Day 1 :
Keynote Forum
Vladimir Torchilin
Northeastern University, USA
Keynote: What properties should nanopreparations possess to become effective anticancer medicines?
Time : 09:30-10:10

Biography:
Vladimir Torchilin is a university distinguished Professor and Director at Northeastern University, Center for Pharmaceutical Biotechnology and Nano-medicine, Boston. He completed his Graduation and MS in Chemistry at Moscow University. He completed his PhD and DSc in Polymer Chemistry and Chemistry of Physiologically Active Compounds in 1971 and 1980, respectively. In 1991, he joined MGH/Harvard Medical School as Head of Chemistry Program, Center for Imaging and Pharmaceutical Research, and Associate Professor of Radiology. He was the Chair in Department of Pharmaceutical Sciences from 1998-2008. His research interests include “liposomes, lipid-core micelles, biomedical polymers, drug delivery and targeting, pharmaceutical nano carriers and experimental cancer immunology”. He has published more than 350 original papers (which received more than 30,000 citations), more than 150 reviews and book chapters.
Abstract:
Tumor therapy, especially in the case of multidrug resistant cancers, could be significantly enhanced by using siRNA down-regulating the production of proteins, which are involved in cancer cell resistance, such as Pgp or survivin. Even better response could be achieved such as siRNA could be delivered to tumors together with chemotherapeutic agent. This task is complicated by low stability of siRNA in biological surrounding. Thus, the delivery system should simultaneously protect siRNA from degradation. Additionally, these nano preparations can be loaded into their lipidic core with poorly water soluble chemotherapeutic agents, such as paclitaxel or camptothecin. In experiments with cancer cell monolayers, cancer cell 3D spheroids, and in animals with implanted tumors, it was shown that such co-loaded preparations can significantly down-regulate target proteins in cancer cells, enhance drug activity, and reverse multidrug resistance. In order to specifically unload such nano preparations inside tumors, we made them sensitive to local tumor-specific stimuli, such as lowered pH, hypoxia, or overexpressed certain enzymes, such as matrix metalloproteases. Using pH-, hypoxia-, or MMP2-sensitive bonds between different components of nano preparations co-loaded with siRNA and drugs, we were able to make the systems specifically delivering biologically active agents in tumors, which resulted in significantly improved therapeutic response.
Keynote Forum
Vladimir Torchilin
Northeastern University, USA
Keynote: What properties should nanopreparations possess to become effective anticancer medicines?
Time : 09:30-10:10

Biography:
Vladimir Torchilin is a university distinguished Professor and Director at Northeastern University, Center for Pharmaceutical Biotechnology and Nano-medicine, Boston. He completed his Graduation and MS in Chemistry at Moscow University. He completed his PhD and DSc in Polymer Chemistry and Chemistry of Physiologically Active Compounds in 1971 and 1980, respectively. In 1991, he joined MGH/Harvard Medical School as Head of Chemistry Program, Center for Imaging and Pharmaceutical Research, and Associate Professor of Radiology. He was the Chair in Department of Pharmaceutical Sciences from 1998-2008. His research interests include “liposomes, lipid-core micelles, biomedical polymers, drug delivery and targeting, pharmaceutical nano carriers and experimental cancer immunology”. He has published more than 350 original papers (which received more than 30,000 citations), more than 150 reviews and book chapters.
<span style="font-size: 12pt; font-family: " times="" new="" roman",="" serif;vladimir="" torchilin="" is="" a="" university="" distinguished="" professor="" and="" director="" at="" northeastern="" university,="" center="" for="" pharmaceutical="" biotechnology="" nano-medicine,="" boston.="" he="" completed="" his="" graduation="" ms="" in="" chemistry="" moscow="" university.="" phd="" dsc="" polymer="" of="" physiologically="" active="" compounds="" 1971="" 1980,="" respectively.="" 1991,="" joined="" mgh="" harvard="" medical="" school="" as="" head="" program,="" imaging="" research,="" associate="" radiology.="" was="" the="" chair="" department="" sciences="" from="" 1998-2008.="" research="" interests="" include="" “liposomes,="" lipid-core="" micelles,="" biomedical="" polymers,="" drug="" delivery="" targeting,="" nano="" carriers="" experimental="" cancer="" immunology”.="" has="" published="" more="" than="" 350="" original="" papers="" (which="" received="" 30,000="" citations),="" 150="" reviews="" book="" chapters.
Abstract:
Tumor therapy, especially in the case of multidrug resistant cancers, could be significantly enhanced by using siRNA down-regulating the production of proteins, which are involved in cancer cell resistance, such as Pgp or survivin. Even better response could be achieved such as siRNA could be delivered to tumors together with chemotherapeutic agent. This task is complicated by low stability of siRNA in biological surrounding. Thus, the delivery system should simultaneously protect siRNA from degradation. Additionally, these nano preparations can be loaded into their lipidic core with poorly water soluble chemotherapeutic agents, such as paclitaxel or camptothecin. In experiments with cancer cell monolayers, cancer cell 3D spheroids, and in animals with implanted tumors, it was shown that such co-loaded preparations can significantly down-regulate target proteins in cancer cells, enhance drug activity, and reverse multidrug resistance. In order to specifically unload such nano preparations inside tumors, we made them sensitive to local tumor-specific stimuli, such as lowered pH, hypoxia, or overexpressed certain enzymes, such as matrix metalloproteases. Using pH-, hypoxia-, or MMP2-sensitive bonds between different components of nano preparations co-loaded with siRNA and drugs, we were able to make the systems specifically delivering biologically active agents in tumors, which resulted in significantly improved therapeutic response.
<span style="font-size: 12pt; font-family: " times="" new="" roman",="" serif;="" tumor="" therapy,="" especially="" in="" the="" case="" of="" multidrug="" resistant="" cancers,="" could="" be="" significantly="" enhanced="" by="" using="" sirna="" down-regulating="" production="" proteins,="" which="" are="" involved="" cancer="" cell="" resistance,="" such="" as="" pgp="" or="" survivin.="" even="" better="" response="" achieved="" delivered="" to="" tumors="" together="" with="" chemotherapeutic="" agent.="" this="" task="" is="" complicated="" low="" stability="" biological="" surrounding.="" thus,="" delivery="" system="" should="" simultaneously="" protect="" from="" degradation.="" additionally,="" these="" nano="" preparations="" can="" loaded="" into="" their="" lipidic="" core="" poorly="" water="" soluble="" agents,="" paclitaxel="" camptothecin.="" experiments="" monolayers,="" 3d="" spheroids,="" and="" animals="" implanted="" tumors,="" it="" was="" shown="" that="" co-loaded="" down-regulate="" target="" proteins="" cells,="" enhance="" drug="" activity,="" reverse="" resistance.="" order="" specifically="" unload="" inside="" we="" made="" them="" sensitive="" local="" tumor-specific="" stimuli,="" lowered="" ph,="" hypoxia,="" overexpressed="" certain="" enzymes,="" matrix="" metalloproteases.="" ph-,="" hypoxia-,="" mmp2-sensitive="" bonds="" between="" different="" components="" drugs,="" were="" able="" make="" systems="" delivering="" biologically="" active="" agents="" resulted="" improved="" therapeutic="" response.
Keynote Forum
Andreas Bernkop-Schnürch
University of Innsbruck, Austria
Keynote: Oral Delivery of Biologics – Back to the Roots
Time : 10:10-10:50

Biography:
Andreas Bernkop-Schnürch was educated in pharmacy (M.Sc.) and in microbiology and genetics (D.Sc.), University of Vienna, finishing his doctorate in 1994. In 2003 he was appointed to a chair in pharmaceutical technology at the University of Innsbruck. Since 2013 he heads the Institute of Pharmacy there. He invented and pioneered thiolated polymers – thiomers – as a new generation of mucoadhesive polymers. Various medicines based on thiomers have already passed clinical trials and a first product will soon reach the global pharmaceutical market. He is the founder of several biotech companies and author of over 300 research articles and reviews. As of June 2016 his H-index is 61.
Abstract:
Within of the last decade biologics became the new, pioneering generation of therapeutics in treatment of numerous diseases. Their fast majority, however, is working through the parenteral route being less accepted and inconvenient, as the oral route for administration of biologics emerged to be problematic mostly due to the enzymatic barrier (I), the mucus gel barrier (II) and the absorption barrier (III) of the GI-tract. To overcome these barriers, a huge variety of strategies were established. Among these different strategies, lipophilic emulsifying delivery systems - having already been established more than 30 years ago for the oral administration of the peptide drug cyclosporine - are nowadays attracting more and more academic and industrial research groups, as the number of encouraging in vivo data and late stage clinical trials is strongly increasing. Among lipophilic emulsifying delivery systems in particular self-emulsifying drug delivery systems (SEDDS) are in focus of research and development. Despite their hydrophilic character biologics can be incorporated in the lipophilic phase of SEDDS via complexation with lipophilic excipients. Once emulsified in the GI-tract to lipid droplets in the size of 30-200 nm, SEDDS provide a protective effect towards a presystemic metabolism without taking the risk of any side effects. Furthermore, SEDDS exhibit comparatively high mucus permeating properties and can be taken up by epithelial cells in an efficient manner. Moreover, SEDDS can be produced very simply and cost effectively. Because of these properties they seem to be a promising tool for oral administration of biologics.
Keynote Forum
Steve Hood
GSK, UK
Keynote: Quantifying the effectiveness of drug delivery systems in vivo
Time : 11:10-11:50

Biography:
Steve Hood completed his PhD in Molecular Toxicology at Surrey University and joined Glaxo Group Research as a Post-Doctoral Fellow in 1993. Following the formation of GlaxoSmithKline (GSK) in 2001, he managed a team determining the drug-drug interaction liabilities while developing ADME strategies for the emerging GSK antibody portfolio. In 2010, he led a cross divisional team charged with understanding and overcoming the delivery issues inherent in GSK’s diverse oligonucleotide portfolio while helping to develop these molecules from early discovery to file. In 2011, he initiated a project that has grown into the active IMI academic/industry COMPACT collaboration (http://www.compact-research.org/) and he is actively involved in facilitating the interaction between academia, biotech and large industry partners.
Abstract:
Keynote Forum
Amiram Goldblum
The Hebrew University of Jerusalem, Israel
Keynote: New Drug Candidates for Liposomal Delivery Identified by Computer Modeling of Liposomes' Remote Loading and Leakage
Time : 11:50-12:30

Biography:
Prof. (emeritus) Goldblum is Head of the Molecular Modeling and Drug Design and Discovery Unit at the Institute for Drug Research of the Hebrew University. Following a BSc in Chemistry and Physics and a MSc in QM studies of molecular spectra, Goldblum's PhD is in Organic Reaction Mechanisms (Hebrew University) followed by Postdoc studies of Quantum Biochemistry (Paris), and of QSAR and QM reaction mechanisms (California). Back at Hebrew U, Goldblum performed research of protein reactions and interactions using semiempirical QM and developed MNDO/H for dealing with H-bonding in relatively large molecular systems. Since 2000, Goldblum's group focuses on applications of his prize winning generic algorithm (ACS "emerging technologies", Washington D.C. 2000) for finding sets of best solutions in extremely complex combinatorial problems. ISE (Iterative Stochastic Elimination) has been applied to protein structure and conformations, to protein-protein and protein-ligand interactions, to molecular properties and to the discovery of drug candidates.
Abstract:
Remote drug loading into nano-liposomes is in most cases the best method for achieving high concentrations of active pharmaceutical ingredients (API) per nano-liposome that enable therapeutically viable API-loaded nano-liposomes, referred to as nano-drugs. This approach also enables controlled drug release. Recently, we constructed computational models to identify APIs that can achieve the desired high concentrations in nano-liposomes by remote loading. We model and predict API suitability for nano-liposomal delivery by fixing the main experimental conditions: liposome lipid composition and size to be similar to those of Doxil® liposomes. On that basis, we add a prediction of drug leakage from the nano-liposomes during storage. The latter is critical for having pharmaceutically viable nano-drugs. The "load and leak" models were used to screen two large molecular databases in search of candidate APIs for delivery by nano-liposomes. The distribution of positive instances in both loading and leakage models was similar in the two databases screened. The screening process identified 667 molecules that were positives by both loading and leakage models (i.e., both high-loading and stable). Among them, 318 molecules received a high score in both properties and of these, 67 are FDA-approved drugs. This group of molecules, having diverse pharmacological activities, may be the basis for future liposomal drug development. .
- Smart Drug Delivery Systems | Vaccine Drug Delivery Systems | Routes of Drug Delivery
Location: London, UK
Session Introduction
Hyungil Jung
Yonsei University, South Korea
Title: Microneedle: the future of pharmaceutical and cosmeceutical delivery systems
Time : 12:30-12:50

Biography:
Hyungil Jung has completed his Ph.D. from Cornell University and his postdoctoral from California Institute of Technology (Caltech). Since then he had received various awards in biotechnology field such as “Outstanding Contributions”, “Best Contribution Award”, “Excellence in Research Award”, “The 31st Industry-academic Cooperation Award”, “Best technology Award”, “Best Teaching Award” and many more because of his outstanding research ability in biotechnology field. Dr. Jung has also recently registered his company, Juvic Inc. to further expand his research and to introduce novel microneedle based pharmaceutical and cosmeceutical products to the market.
Abstract:
Microneedles are microscopic needles capable of delivering pharmaceutical compounds, proteins and even cosmetics into the skin in a minimally invasive manner. There are various types of microneedles like solid, hollow and dissolving microneedle. Each of these microneedles, based on the application purposes can be applied in different branches of drug or cosmetic compounds delivery. Through microneedles, achievement of a highly efficient delivery has become possible and we expecting microneedles to replace the widely used hypodermic needles in near future. Rather than the drug delivery, microneedles can be applied in cosmetics field like anti-wrinkles, whitening or even anti-ageing. To develop a microneedle system that can fully replace hypodermic needles, we should focus on solving the limitations of current microneedle system like loading amount limitation, delivery time limitation, application limitation etc. In our laboratory at Yonsei University, and at Juvic Inc., we have already solved the main limitations of traditional microneedle systems through various patented technologies.
Steve Rannard
University of Liverpool, UK
Title: Solid drug nanoparticles as oral and long acting parenteral drug delivery for infectious diseases
Time : 12:50-13:10

Biography:
Steve Rannard is a materials chemist at the University of Liverpool (UoL) where he holds a personal Chair in the Department of Chemistry. He is the academic lead for Nanomedicine within the Materials Innovation Factory and Director of the Radiomaterials Laboratory. Steve spent 16 years in industry (Cookson, Courtaulds, Unilever) and has co-founded three start-up companies (IOTA NanoSolutions Ltd, Hydra Polymers Ltd and Tandem Nano Ltd). Since returning to academia in 2007 his collaborative grant income from funders including MRC (UK), EPSRC (UK), NIH (US), USAID, CRUK, CHAI, and BSAC (UK) and industry has exceeded £16m and his current research focuses materials science onto unmet medical/clinical needs to target new patient benefits using scalable polymer nanoparticle synthesis, solid drug nanoparticle formulation and nanoemulsion platforms.
Abstract:
Nanomedicine has focused heavily considerably on acute disease over several decades; however, there is considerable clinical need for new interventions for infectious disease prevention and therapy that allows patients to manage currently life-long conditions. Oral dosing is the only widely patient-acceptable administration format for chronic disease as daily, or more frequent, injections are not well tolerated. For prevention of disease and long term therapy, adherence to dosing regimens is critical to either maintain control or maintain protection over long periods. Here, we have generated a new approach to solid drug nanoparticle (SDN) formation and rapid candidate therapy identification that allows 1000’s of nanoparticle options to be generated and accelerated through a series of pharmacological tests to establish potential benefits. Two case studies will be presented, namely a candidate for reduced oral dose HIV therapy and a prophylactic antimalarial injection that provides long-term protection to infection. Methodology & Theoretical Orientation: Solid drug nanoparticle candidates were generated using an accelerated emulsion-templated freeze drying (ETFD) screening approach in both cases of HIV and malaria nanomedicine production. “Hits” were selected based on their chemical performance and progressed to a series of pharmacological studies that characterized a number of relevant factors. “Leads” were selected based on their pharmacological potential and, in the case of HIV candidate nanomedicines, translated from ETFD screen manufacture to cGMP production using emulsion spray drying (EFD). Powdered products from EFD were hand filled into capsules for human healthy volunteer evaluation. Findings: Oral dosing of two HIV antiretroviral SDNs has shown the potential for a 50% reduction of the dose of drug within daily regimens containing efavirenz or lopinavir. In the case of antimalarial prophylaxis, an intramuscular depot injection of SDNs has been shown to produce a minimum of 28 day protection in a mouse model, offering possible long-term protection in future human studies. Conclusion & Significance: Combined and systematic solid drug nanoparticle screening by both materials chemistry and pharmacology allows rapid identification of new nanomedicine candidates for diverse diseases with the potential for rapid translation to clinic.
Achim Aigner
Leipzig University, Germany
Title: Polymeric nanoparticles for therapeutic siRNA delivery: analysis of tissue-penetration and biological activities in tumor tissue slice cultures and in vivo xenograft models
Time : 14:00-14:20

Biography:
Achim Aigner is Professor for Clinical Pharmacology with research interests in the (preclinical) development of novel therapeutic strategies based on small RNA molecules (siRNAs, miRNAs, antimiRs) in oncology. One major focus is on the development and evaluation of polymer-based nanoparticles for in vivo use, including chemical modifications. Systems are tested in various in vitro, ex vivo and in vivo models of solid tumors. To this end, different target genes (established and novel oncogenes) as well as tumor inhibitory miRNAs or the inhibition of oncogenic miRNAs are explored.
Abstract:
The efficient delivery of small RNA molecules like siRNAs or miRNAs still represents a major hurdle in their therapeutic application for gene knockdown or miRNA replacement. Polymeric nanoparticles e.g. based on low molecular weight polyethyleneimines (PEIs) have been successfully explored, and chemical modifications further increase efficacy and improve biocompatibility. Among those, promising strategies include the modification of PEI with amino acids like tyrosine, yielding low molecular weight Tyr-PEIs (PxY with x = 2kDa, 5kDa, 10kDa), or the combination of PEI-based polyplexes with liposomes, resulting in lipopolyplexes. Both systems demonstrate improved in vitro properties and excellent applicability in vivo, as shown in mouse tumor xenograft models.
The therapeutic success of nanoparticles depends, among others, on their ability to penetrate a tissue for actually reaching the target cells, and their efficient cellular uptake in the context of intact tissue and stroma. Thus, beyond rather artificial tissue culture or rather tedious in vivo models, efficient ex vivo systems closely mimicking in vivo tissue properties are needed.
We have established tumor tissue slice cultures for the analysis of tissue-penetrating properties and biological activities of nanoparticles. As a model system, we employed slice cultures from different tumor xenograft tissues for analyzing modified or non-modified PEI/siRNA complexes and their lipopolyplex derivatives. Excellent tissue preservation was observed for >14 days, thus allowing for prolonged experimentation and analysis. Fluorescence microscopy of cryo-sectioned tissue slices shows different degrees of nanoparticle tissue penetration, dependent on their surface charge. More importantly, the determination of siRNA-mediated knockdown efficacies of endogenous target (onco-) genes reveals the possibility to accurately assess biological nanoparticle activities in situ, i.e. in living cells in their original environment. Thus, we introduce tumor (xenograft) tissue slices for the facile ex vivo assessment of important biological nanoparticle properties in a relevant setting.
Fiorenza Rancan
Universitätsmedizin Berlin, Germany
Title: Drug delivery across skin barrier: Investigation on different biocompatible thermoresponsive soft nanocarriers
Time : 14:20-14:40

Biography:
Fiorenza Rancan expertise lies in the use of human skin explants and skin organ culture as model for skin penetration and drug delivery studies. Within the last years she extensively investigated nanocarrier skin interactions with focus on both biological properties and toxicological effects of particle-based systems. Main research topics are the use of biodegradable particles (e.g. PLA and virus-like particles) for the delivery of adjuvants and antigens to skin (transcutaneous vaccination), the exploration of new generation nanocarriers for the treatment of skin inflammatory conditions giving special attention to antigen presenting cells, and the development of new antimicrobial treatments using skin models for infected chronic wounds.
Abstract:
Despite skin accessibility, delivery of drugs across skin barrier and the maintenance of a constant drug concentration in the target region is still a challenge. Nanocarrier-based approaches have been shown to improve both dermal and transdermal drug delivery. Depending on the type of nanocarrier, different drug release properties as well as different interactions with skin barriers and cell components can be achieved. A systematic correlation between nanocarrier physicochemical characteristics and their skin penetration and drug delivery properties is necessary to foster the use of nanotechnology in dermatology. Nanocarriers physicochemical properties and their performance after application on human skin explants have been investigated by methods like atomic force microscopy and stimulated Raman spectroscopy, whereas fluorescence and electron microscopy as well as flow cytometry of single skin cells served to elucidate nanocarrier penetration pathway and cellular uptake. Results show that size, surface charge, type of cargo, softness, and stability mostly influence nanocarrier penetration and drug delivery to skin. In particular, thermoresponsive nanogels which can release loaded drugs preferentially above a distinct temperature, represented an attractive approach to improve the selectivity of anti-inflammatory therapies. In addition soft, thermoresponsive nanogels were found to penetrate deeply within the stratum corneum, the outermost skin barrier, changing its permeability and improving drug penetration. Penetrated nanogels were internalized by skin cells in both epidermis and dermis. Interestingly, also significant percentage of antigen presenting cells were found to be associated with nanocarriers depending on the degree of skin barrier disruption. These observations could further be developed for specific targeting approaches in order to increase drug delivery to key cell populations.
Patrizia Chetoni
University of Pisa, Italy
Title: Investigation on nano-sized drug delivery systems for ocular application
Time : 15:00-15:20

Biography:
Patrizia Chetoni earned her PhD in Pharmaceutical Sciences in 1991 at the University of Pisa and she is currently Associate Professor of Pharmaceutical Technology at the Department of Pharmacy of the same University. She has conducted extensive researches in the technologies for drug delivery. In particular, she has worked in the development of strategies to improve absorption of drugs through biological barriers (cornea, skin, buccal mucosa and nail) and in the development of cell cultures models for prediction of drug bioavailability and of their cytotoxicity. She has also studied animal models to determine the bioavailability of topically applied drugs and experimental methods for the characterization of mucoadhesive properties of drug delivery systems. She has published more than 70 research papers in international referred journals she has also contributed to some book chapters and international patents.
Abstract:
The unique characteristics of the eye and the presence of strong defence mechanisms make difficult to achieve therapeutic concentrations of drug in the different districts of the eye after topical instillation of eye-drop. One of the main challenge to increase the poor ocular bioavailability of conventional formulations is to improve the low drug-contact time by reducing drainage, tear turnover and dilution or lacrimation. In addition, another strategy is to enhance the drug penetration across the cornea, which represents an effective barrier to drug permeation due to the presence of the annular tight junctions on corneal epithelium. Various drug delivery systems have been developed to increase the bioavailability of ophthalmic drug. In particular, nano-sized carriers like liposome, nanoparticle, SLN and nanomicelle have gained wide interest, providing an increase in the pre-corneal residence time, mucoadhesion and penetration across the eye tissues of drugs. Conventional delivery systems usually require administering at regular time intervals, whereas nano-sized carriers often release drugs at constant rate for a prolonged period of time and thus enhance their absorption and promote a site specific delivery especially when dispersed or suspended in polymer solutions with mucoadhesion properties. In fact, when applied topically as eye-drop, liposomes can attach to the hydrophobic corneal epithelium, where they continuously release the encapsulated drug, improving pharmacokinetics behaviour and decreasing toxic side effects of encapsulated drugs. Polymeric nanoparticles protect drug from metabolic degradation and interact strongly with both ocular surface and drug, more than the same mucoadhesive polymer in solution. SLN for their lipophilic nature, favour drug permeation through the highly hydrophobic corneal epithelium and furthermore, transscleral route might contribute to drug absorption in vitreous humour. The consistent progress in formulation efficiency of nano-sized carriers will be described in view of their application in ophthalmic field.
Balint Sinko
Pion Inc., USA
Title: Application of simultaneous dissolution-absorption apparatus for screening formulations before bioequivalence studies
Time : 15:00-15:20

Biography:
Bálint Sinkó received M.Sc. degree in Pharmacy at Semmelweis University in 2007. As a graduate student and a research fellow of Krisztina Takács-Novák his work focused on chemical analysis of Pharmaceutical formulations. He has started his Ph.D. in the same group in 2007 taking part in the installation of a new permeability lab. During his research he has developed a PAMPA model for the prediction of skin penetration that formed the main part of his Ph.D. thesis. He received his Ph.D. degree in 2012, while the developed Skin PAMPA model has been licensed to Pion Inc in the same year. After PhD he has joined Pion Inc. where he currently works as manager of technology development and support.
Abstract:
For generic drug development traditional dissolution tests have been used in the pharmaceutical industry to compare performance of different drug product formulations before conducting bioequivalence studies, even though the in vivo predictive power of these tests are questionable. When a poorly water-soluble API is formulated to enhance its dissolution, additives have an effect not only on dissolution, but also on flux through the membrane. The aim of this study was to demonstrate that a simultaneous dissolution-absorption test can be used as a predictive tool before bioequivalence studies are conducted. Telmisartan tablets were tested using MacroFLUX. Receiver chamber integrated with permeation membrane, overhead stirrer and UV probe was inserted in the standard 900 mL vessel of USP II apparatus.
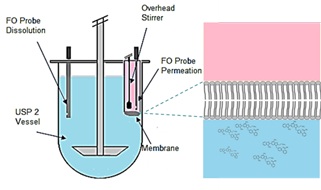
An artificial membrane with 3.8 cm2 area was separating the dissolution compartment from the receiver compartment containing 15 mL of pH7.4 buffer. The dissolution and flux results of the brand name (Micardis) and generic (Actavis) Telmisartan 40 mg tablets were compared. Actavis showed a slower release kinetics than Micardis, though reached the same maximum concentration after 110 min. The flux from the generic product was found to be 0.240 ± 0.011 µg/(cm2*min), which is only 71% of the flux of the brand name (0.337 ± 0.028 µg/(cm2*min)). This in vitro result showed excellent correlation with the in vivo data from bioequivalence studies, where the appearence rate or the drug in blood from Actavis was 72 % of the rate from Micardis. The in vivo predictive power of the simultaneous dissolution-absorption test was demonstrated by comparing the in vitro fluxes to in vivo rate of appearance in blood of brand name and generic formulation of telmisartan.
Christine Dufès
University of Strathclyde, UK
Title: Tumour regression after intravenous administration of novel tumour-targeted nanomedicines
Time : 15:20-15:40

Biography:
Christine Dufès is a Senior Lecturer at the Strathclyde Institute of Pharmacy and Biomedical Sciences (SIPBS), University of Strathclyde, Glasgow, United Kingdom. She obtained a Doctorate in Pharmacy (with Distinction and congratulations of the Jury) and a PhD (with a European Label, Distinction and congratulations of the Jury) from the University of Poitiers (France). After four years as a post-doctoral researcher at the Cancer Research UK Beatson Laboratories in Glasgow, she was appointed as a Lecturer at SIPBS in 2006, obtained fellowship of the Higher Education Academy in 2007 and became a Senior Lecturer in 2012. Her research interests include the targeted delivery of drugs and therapeutic genes to tumours and cerebral diseases. She has been awarded the Biochemical Journal Young Investigator Award (2009) and the Tom Gibson Memorial Award (2012) for her research, in addition to the Best Overall Strathclyde Teaching Excellence Award 2013 for her teaching. She sits on the editorial boards for 17 journals.
Abstract:
The possibility of using genes as medicines to treat cancer is limited by the lack of safe and efficacious delivery systems able to deliver therapeutic genes selectively to tumours by intravenous administration, without secondary effects to healthy tissues. In order to remediate to this problem, we investigated if the conjugation of the generation 3 diaminobutyric polypropylenimine dendrimer to transferrin and lactoferrin, whose receptors are overexpressed on numerous cancers, could result in a selective gene delivery to tumours after intravenous administration, leading to an increased therapeutic efficacy. The intravenous administration of transferrin-bearing and lactoferrin-bearing polypropylenimine dendriplexes resulted in gene expression mainly in the tumours. Consequently, the intravenous administration of the transferrin-bearing delivery system complexed to a therapeutic DNA encoding tumour necrosis factor (TNF)α led to 90% tumour suppression over one month on A431 epidermoid tumours. It also resulted in tumour suppression for 60% of PC-3 and 50% of DU145 prostate tumours. Furthermore, the intravenous administration of the lactoferrin-bearing targeted dendriplexes encoding TNFα led to the complete suppression of 60% of A431 tumours and up to 50% of B16-F10 skin tumours over one month. Transferrin- and lactoferrin-bearing polypropylenimine dendrimers are therefore highly promising delivery systems for cancer therapy.
- Nanotechnology in Drug Delivery | Pharmaceutical Nanotechnology | Biomaterials in Drug Delivery
Location: London, UK
Session Introduction
Flavia Laffleur
University of Innsbruck, Austria
Title: Biomaterials a pathway to overcome biomembranes
Time : 16:00-16:20

Biography:
Flavia Laffleur, is a senior researcher of Drug Delivery in the Department of Pharmacy at LFU Innsbruck, Austria. Flavia Laffleur published over 55 publications and gave oral presentations on several international conferences. From 2010 until 2013 she completed her doctoral thesis focused on smart drug delivery systems. Since 2013, she is a senior researcher at the Department of Pharmaceutical Technology in Innsbruck. Since 2017, Dr. Flavia Laffleur is a researcher at the MIT, in Boston, Massachusetts. She received several awards, including Lesmüller-Stiftung award and the Galenus Foundation Technology Award. Currently Dr. Laffleur´s research focusses on mucosal drug delivery as well as smart delivery systems to overcome biological barriers
Abstract:
Dry eye – a disease affecting between 4 and 34 % of the population worldwide. Stressful conditions to ocular surface, contact lenses, systemic disease (e.g. antidepressants, thyroid disease and diuretics cause dry eye. Complaints are dryness and tear film instability as well as evaporation caused by ocular surface changes. Therefore, it was aimed to investigate novel synthesized hyaluronic acid derivate evaluating its potential in mucoadhesion and lubricant for the treatment of dry eye syndrome. Hyaluronic acid, a well-known biomaterial in the ocular delivery was chemically modified with cysteine ethyl ester (HA-CYS). HA-CYS was evaluated in terms of mucoadhesive strength on ocular mucosa. Stability measurements and lubricative assay were conducted in form of disintegration and water uptake capacity, respectively. Moreover, safety consideration proceeded with in vitro cell line. Most important Hen's Egg Test on the chorionallantoic membrane for the mucous membrane compatibility was evaluated. According to the results HA-CYS achieved due to this thiolation more pronounced mucoadhesive, stability and lubricative properties enhanced. 3.81-fold increased swelling capacity, 30.5- fold more improved mucoadhesive properties and 9.72-fold higher stability of hyaluronic acid was achieved due to the chemical modification. Thus, the promising results underpin further exploitation of this versatile polysaccharide for treating dry eye syndrome
António José Ribeiro
University of Coimbra, Portugal
Title: Nanoprecipitation for delivery of Insulin
Time : 16:20-16:40

Biography:
Antonio Ribeiro is a Professor of Pharmaceutical Technology at the Faculty of Pharmacy of University of Coimbra where he managed a high international reputed research group. He has a Ph.D Degree in Pharmaceutical Development and Biopharmacy and his research has been focused on design of delivery systems for peptidic and protein drugs. He has published more than sixty peer-reviewed publications, among which several very highly cited, and he has been a keynote speaker and presented various talks all over the world. He is also an expert and consultant in intellectual property of drugs for pharmaceutical industry. He serves as an Editorial member of several publications and as a consultant for several research agencies mostly related to Diabetes and Nanotechnology.
Abstract:
Micro and nanoparticulates made of poly (lactic-co-glycolic acid) (PLGA) have been extensively studied due to their biocompatibility, biodegradability and ability to control release of drugs and have been linked to delivery of proteins. Nanoprecipitation is the most appropriate method to produce nano sizing particles for parenteral delivery without harsh conditions of temperature and agitation, making use of water miscible solvents and involving a simple set up. However, attempts to encapsulate proteins and specially insulin in PLGA nanoparticles using nanoprecipitation have revealed limited success due to a low efficiency of encapsulation /EE). Insulin-PLGA nanoparticles (Ins-PLGA NPs) for parenteral administration were produced by nanoprecipitation without any co-solvent or additive to insulin, buffering the dispersant phase. NPs were freeze-dried with sorbitol and characterized for size and polydispersity (PdI) and zeta potential. Insulin extracted from NPs was assayed using HPLC and its conformation was assessed before, during and after procedure using circular dichroism (CD). In vitro release studies were performed to access insulin release kinetics.
NP's with a mean size lower than 200 nm a low PDI were obtained after freeze-drying and revealed physical stability after reconstitution in water. EE of NP's was greatly improved compared to previous attempts, and among formulations, the choice of a buffer with a pH close to the PI of insulin revealed a higher EE. Insulin secondary conformation was maintained during manufacture so insulin was not markedly degraded during the manufacturing process. Insulin release from NP's showed a high burst effect and a release medium pH behavior. The PLGA based NP's buffered formation occurs under mild conditions and consequently can be used as a platform for delivery of labile molecules such as most of the biotechnology-based drugs.
Serena Mazzucchelli
University of Milan, Italy
Title: Metronomic treatment of breast cancer with Doxorubicin-loaded ferritin nanocages prevents chemoresistance and cardiotoxicity in comparison to liposomal Doxorubicin
Time : 16:40-17:00

Biography:
Serena Mazzucchelli, PhD, research associate at the University of Milan (UNIMI). Bachelor degree in Biological Sciences (2004), degree in Biology (2006) and PhD in Biological Sciences (2009) at the Department of Biotechnology and Biosciences (University of Milan-Bicocca-Italy). From 2009 to 2012 she has a post-doc fellowship at the Department of Biomedical and Clinical Sciences “L. Sacco” (DIBIC-UNIMI). Until 2015 she is researcher at the “L. Sacco” University Hospital. Today, SM is research associate of the Nanomedicine Laboratory carrying out her research focused on the development of nanodevices therapy of breast cancer at the DIBIC-UNIMI. She is an author of 35 papers and a reviewer.
Abstract:
Metronomic chemotherapy (LDM) is based on frequent drug administrations at lower doses, resulting in neovascularization inhibition and induction of tumor dormancy. LDM application in clinical practice is limited by: 1) low drug accumulation at tumor site, 2) controversial effectiveness against chemoresistance in advanced metastatic cancers, and 3) acquired resistance after prolonged treatment.
Nanotechnology could offer groundbreaking solutions to improve the effectiveness of LDM chemotherapy, by taking advantage of the unique targeting efficiency of ferritin (HFn) nanocages. Here, we exploit the HFn mediated targeted delivery of doxorubicin (DOX) in an aggressive breast cancer mouse model with DOX inducible chemoresistance.
HFnDOX was recently demonstrated to overcome chemoresistance by actively promoting DOX nuclear translocation in vitro and was tested as a MTD treatment on a DOX sensitive tumor model with encouraging results. We find that LDM administration of HFnDOX strongly improves the antitumor potential of DOX chemotherapy arresting the tumor progression, demonstrating that HFn mediate the nuclear delivery of DOX and increase its accumulation both in tumor tissue and in cancer cell nuclei. Moreover, we find that HFnDOX antitumor effect is attributable to multiple nanodrug actions beyond cell killing, including inhibition of tumor angiogenesis and avoidance of chemoresistance. Otherwise, although an even better reduction of tumor progression was achieved with liposomal DOX (plDOX), a fivefold increase in MDR1 positive cells has been displayed, suggesting that plDOX is not suitable in view of a protracted LDM treatment, due to the onset of chemoresistance. Multiparametric assessment of hearts, including histology, ultrastructural analysis of tissue morphology, and measurement of markers of reactive oxygen species and hepatic/renal conditions, provided evidence that metronomic HFnDOX allowed us to overcome cardiotoxicity contrary to what is observed with DOX and plDOX.
Our results suggest that HFnDOX has tremendous potential for the development of “nanometronomic” chemotherapy toward safe and tailored oncological treatments.
Victoria Sherwood
University of Dundee, UK
Title: An early developmental vertebrate model to assess nanomaterial safety
Time : 17:00-17:20

Biography:
Victoria Sherwood is a Discovery Fellow in Skin Cancer Biology at the University of Dundee. Dundee is ranked number one for biological research in the UK. Victoria’s research interests lie in how skin tumours progress into the most dangerous, metastatic forms of the disease and in the development of novel therapeutic strategies to treat these advanced tumours. This has led to an interest in my lab in developing targeted, theranostic NPs for the treatment of metastatic melanoma (one of the most aggressive forms of skin cancer). As part of this on-going work my lab have invested efforts in improving techniques to assess NP safety, as nanotoxicity is often a major barrier to the clinic for nanoformulations. Here I will present some of our published work to address these issues and will discuss future directions that we are taking in the lab to further improve safety assessment of nanotherapeutic materials.
Abstract:
Statement of the Problem: We are developing targeted, drug-loaded nanocarriers for treating the most aggressive forms of skin cancer (Baldelli Bombelli et al., 2014). These nanoformulations are complex, multicomponent drug delivery devices, which due to their high surface area-to-volume ratio and complexity in the materials used for construction, have the potential to result in toxicity when administered to patients. Thus, robust safety assessment is a major concern when developing such nanotherapeutic agents for the clinic.
Methodology & Theoretical Orientation: We have developed a system to rapidly and robustly assess nanoparticle (NP) safety, early on in the development process of novel nanotherapeutics (Webster et al., 2016; Al-Yousuf et al., 2017). This enables us to optimize the NP design and/or synthesis protocol in order to obtain nanotherapies that are safe for use in patients. Cell-based assays are the most commonly used approach for nanotoxicity assessment, but these methods are known to provide poor in vitro-in vivo correlations. We have incorporated an early developmental vertebrate phenotypic screening assay using Xenopus laevis as a model organism, into our nanotoxicity assessment protocol, to complement the cytotoxicity data. Combining data from these two nanotoxicity assessment approaches, provides an overall NP hazard assessment index. This index can inform researchers whether or not to progress with further assessment of NP safety in expensive, more labor/time intensive mammalian models, or to first refine the nanoformulation (Figure 1).
Findings: Using this approach, we assessed NP safety using a variety of nanomaterials (including formulations developed for biomedical applications). The approach could predict NP safety as confirmed through in vivo assessment in mice.
Conclusion & Significance: This work highlights the potential of early developmental models as a rapid screening tool for nanomaterial safety and suggests that such models could be incorporated into routine nanotoxicity assessment protocols.
Dimitrios A. Lamprou
University of Kent, UK
Title: Polymeric nanofibers for controlled release in hernia repair
Time : 17:20-17:40

Biography:
Dimitrios Lamprou (BEng, PgCert, PgDip, MSc, PhD, and MBA) is Associate Professor in Pharmaceutics & Drug Delivery at University of Kent (UK) and Visiting Academic at University of Strathclyde (UK). Lamprou has been trained in multidisciplinary areas, worked in first class laboratories, and has experience of teaching in Higher Education, conducting research and securing National and International Funding (over £1.5M). Dr Lamprou has authored over 50 articles in high impact multidisciplinary journals, and over 150 poster and podium presentations - that includes over 50 invited talks.
Abstract:
A hernia of the abdominal wall is a permanent or intermittent protrusion of abdominal contents outside the abdominal cavity through a defect in the abdominal wall. Hernia can be congenital or acquired, the latter mainly being as a result of the incision made during surgery. Many hernias can result in no symptoms, however some can go on to develop a range of problems from pain and cosmetic appearance to bowel obstruction, fistula and bowel ischaemia, which can be life-threatening. The majority of hernias are repaired by inserting a mesh to stabilise the weakness in the abdominal wall. These meshes can be biological (e.g. porcine) or synthetic. However, despite many different approaches to repair hernia with mesh, many hernias go on to recur or result in complications themselves (mesh erosion into surrounding organs; adhesions for example), increasing the potential symptoms and morbidities for the patient involved. The purposes of this study was to analysed the physical and physiological properties of an abdominal wall hernia and to develop a biological scaffold in order to overcome the physical and physiological limitations of abdominal wall hernia. Two advanced fabrication techniques (Electrospinning and 3D Printing) was used for the formulation of the scaffolds with and without drug molecules, in order to obtain a system that can facilitate hernia repair and wound healing. The systems was characterised by state-of-the-art techniques such as AFM, ToF-SIMS, CAG, Rheology.
Reno A L Leon
Structo Pte. Ltd., Singapore
Title: Multi-component orchestrated delivery modules for personalized healthcare using SLA 3D printing
Time : 17:40-18:00

Biography:
Reno A. L. Leon has completed his postdoctoral studies from National University of Singapore and is currently the CSO-Materials at Structo Pte. Ltd., Singapore. He has 4 years of industrial experience in polymeric materials for novel drug delivery, digital dentistry and micro-formulations. He has 2 patents and 5 peer reviewed publications to his credit. His research interests include 3D delivery systems, personalized healthcare solutions, 3D bioprinting and digital therapeutics.
Abstract:
Therapeutic delivery has long been the crux of medical advancement due to its direct affiliation with the patient. However the technological pathway hasn’t matched up with the growing demands of customizability and compliance. Unfortunately the present state of therapeutic prescription is at a standstill causing longer batch hours, huge stock piling, logistics cost, medication tracking and all this leading to enormous amounts of cost, man hours and compliance. Overarching all of the above is that the prescriptions are bulk manufactured with therapeutic amounts assigned based on averaged clinical data.
Here we report a first demonstration of customizable multi-modal delivery in tandem, ‘The Synco-Orchestration Delivery Module (SODM)’ using 3D printing. The objective of our work is to demonstrate an SLA 3D printed SODMs to address personalized drug delivery. Furthermore, SODMs are designed with multiple compartments to demonstrate multiple API delivery simultaneously. SODMs address most of the drawbacks of traditional delivery systems by bridging the gap between formulation, delivery and design. Significant advantages of SODMs include personalized dosage regimen, reduced number of intakes, programmed release kinetics and on-the-fly printable therapeutics. SODM thus promises a way to completely digitalize personalized delivery in the near future.
Dagmar Fischer
Friedrich-Schiller-University, Germany
Title: Bacterial nanocellulose as controlled drug delivery system in skin applications
Time : 11:50-12:30

Biography:
Dagmar Fischer, a pharmacist by training, has more than 20 years of experience in the field of nanocarriers based on synthetic and natural polymers, their formulation and biopharmaceutical characterization. Furthermore, she has long-standing successful cooperations with many partners in and outside of Europe, in the field of nanosafety. After receiving her PhD and Habilitation at the University of Marburg, she joined for several years a biotech company as Head of Preclinical Research and Development. In 2008 she was appointed as Professor of Pharmaceutical Technology at the University Jena.
Abstract:
The natural hydropolymer bacterial nanocellulose (BNC) is an innovative biomaterial, produced during fermentation by strains of Gram-negative bacteria Komagataeibacter xylinus and consisting of about 1% cellulose and 99% water. Although the chemical formula is identical to plant cellulose, the material favours totally different, but outstanding material characteristics due to the three-dimensional network of nano-sized fibres. The interest in BNC as drug delivery system dramatically increased during the last years, as the nano-sized 3D-network of BNC is expected to hold a large amount of drug molecules due to its large surface area. However, the highly hydrophilic character, limited a broad application, especially for the delivery of lipophilic drugs as well as long-term applications. We developed different loading techniques to accomplish a controlled release of drugs from several hours to weeks using BNC produced under lab-scale as well as under high throughput conditions. Native BNC, hybrid systems with different types of the thermo-responsive block-copolymers Poloxamers as well as lipid-modified BNC were established. Depending on the type of modification, not only the drug release profile, but also superior material properties such as high compression stability and water binding could be achieved. Using the antiseptic octenidine as model drug, the antimicrobial activity against Staphylococcus aureus and Pseudomonas aeruginosa was not changed by the use of the modified BNC. Excellent biocompatibility of the loaded BNC could be demonstrated after local administration in a shell-less hen’s egg model. In conclusion, controllable short- and long-term delivery systems consisting of Poloxamer and lipid modified BNC could be developed as ready-to-use systems e.g. for dermal wound treatment, cosmetics or the use as implants.
