Day 2 :
Keynote Forum
Arwyn T Jones
Cardiff University. UK
Keynote: Overcoming Cellular Barriers for Drug Delivery: Opening Endocytic Gates and Pathways for Intracellular Targetting
Time : 09:30-10:10

Biography:
Arwyn gained his PhD in protein biochemistry and crystallography at Birkbeck College, University of London. Then he undertook postdoctoral positions investigating endocytosis at the University of Liverpool and Harvard University, Boston USA. In 2000 he was awarded a European Molecular Biology Organization fellowship to work at the European Molecular Biology Laboratory (EMBL), Heidelberg Germany, and continued at the EMBL when he was awarded an Alexander von Humboldt Foundation Scholarship. He was appointed as Lecturer at the Cardiff School of Pharmacy and Pharmaceutical Sciences at Cardiff University in 2002 where he is now a Professor in Membrane Traffic and Drug Delivery.
Abstract:
Targeting a disease process inside a cell with biopharmaceuticals still represents a major challenge, not least in overcoming biological barriers such as those posed by the plasma membrane. Investment in this approach is justified when one considers the number individual intracellular targets now available to us as we continue to understand disease processes at the gene and protein level. This is true for many high-burden diseases including cancer, infectious diseases and inherited genetic defects such as cystic fibrosis. Our research is focused on studying endocytosis and specifically on designing methods to analyse individual endocytic pathways to characterise how drug delivery vectors and associated therapeutics gain access to cells. As vectors we have paid particular attention to natural ligands, cell penetrating peptides and antibodies, focusing on their capacity to not only interact with, and enter cells, but then on monitoring their intracellular traffic to reach a final destination. In this lecture I will describe work we have performed focusing on design and characterization of methods to study endocytosis of drug delivery vectors and on recent studies showing how internalisation of plasma membrane receptors can be significantly enhanced, and their normal endocytic routes modified to reach a desired intracellular location. Our involvement in a €30M FP7 Innovative Medicine Initiative (IMI-EFPIA) consortium (COMPACT www.compact-research.org/) will also be discussed. This represents a public-private collaboration between 14 European academic institutes and pharmaceutical companies aiming to improve the cellular delivery of biopharmaceuticals across major biological barriers of the intestine, lung, blood brain barrier and skin.
Keynote Forum
Raid Alany
Kingston University, UK
Keynote: Age-related sight loss: novel drug delivery strategies to the anterior and posterior segments of the eye
Time : 10:10-10:50

Biography:
Professor Raid Alany has over 25 years of international experience in pharmacy education, pharmaceutics and drug delivery research. His academic journey spans three continents, namely, Asia, Oceania and Europe. He received his PhD in drug delivery from the University of Otago, Dunedin New Zealand in 2001; was appointed as a Lecturer at the School of Pharmacy, The University of Auckland, Auckland, New Zealand. He joined Kingston University London as Professor (Chair) of Pharmaceutics in January 2011 and was appointed as Research Director for the School of Pharmacy and Chemistry in December 2013. Raid is an author on over 200 scientific research publications (papers and abstracts), a book and seven book chapters. Professor Alany acts as Editor-in-Chief for Pharmaceutical Development and Technology, Section Editor for Clinical and Experimental Ophthalmology the official journal of the Royal Australian and New Zealand College of Ophthalmologists; Chief Patron, Drug Development and Therapeutics, a publication of Organization of Pharmaceutical Unity with BioAllied Sciences (OPUBS). He serves on the Editorial Board of the following journals: Current Medical Research and Opinion, BioMed Research International, Journal of Drug Delivery Science and Technology, Current Drug Delivery, Pharmaceutics MDPI, and Drug Delivery Letters. He is the Immediate Past President of the New Zealand Chapter of the Controlled Release Society (NZCRS), Young Scientist Committee of the Controlled Release Society. Raid won several awards such as Microscopy New Zealand Young Scientists Award in 1999 The University of Auckland's Vice Chancellor's Early Career Research Excellence Award in 2003, the Controlled Release Society Veterinary Programme co-chair/ chair Distinguished Service Awards in 2008/2009 and the Spark Ideas Challenge, Uniservices Prize and Chiasma Prize in 2011. He consults for human and veterinary pharmaceutical companies in New Zealand and Singapore and is an inventor on several international patents.
Abstract:
Aging is associated with drastic optical and biochemical changes in the eye often leading to a decline in visual acuity where vision worsens. Such eye disorders impose a financial burden on the health sector worldwide. Recent estimates of the global cost of sight loss -up to the year 2010- suggest an annual figure of over US$3 trillion (£2.4 trillion). The main disorders leading to sight loss are cataract, glaucoma, age-related macular degeneration (AMD) and diabetic retinopathy. Pharmaceutical formulation and drug delivery research has introduced promising eye treatments into the market; nevertheless, there remain unmet clinical needs and limitations associated with performance of conventional eye drops and ointments. Compromised adherence and/or persistence with conventional eye drops that are applied topically to the surface of the eye is primarily related to the need to be applied once, twice (or even up to four times) daily, often as a combination of multiple drugs, to achieve their intended purpose. The intravitreal injection of anti-vascular endothelial growth factor (VEGF) for AMD treatment requires clinical intervention every 4-8 weeks. Therefore, achieving therapeutics drug concentrations at the target site and maintaining such concentration over extended time intervals with minimal undesirable effects, offer renewed opportunities for product research and development, especially when using already approved drugs with well-established safety and efficacy profiles. This talk will review and provide insights withdrawn from our own research on ophthalmic drug delivery systems that are aimed at age-related eye disorders including phase-transition microemulsions, in-situ gels, polymeric and inorganic nanoparticles, personalised ocular inserts and modified contact lenses.
Keynote Forum
Sharareh Salar-Behzadi
Research Center Pharmaceutical Engineering GmbH, Austria
Keynote: Lipid-based pharmaceutical formulations for patient-centric product development
Time : 11:10-11:50

Biography:
Sharareh Salar-Behzadi held her diploma in Pharmacy and PhD in Pharmaceutical Technology from University of Vienna. Her experience covers a broad range, including formulation and process development for production of solid dosage forms. She worked on several pharmaceutical manufacturing methods, among them solvent free hot-melt fluid-bed technology, wet fluid-bed granulation, roller compaction and methods for development of nano lipid carriers. She works at Research Center Pharmaceutical Engineering (RCPE) GmbH since 2012 as Project Lead for scientific execution of projects for formulation engineering and development of particulate dosage forms. An important research focus is development of personalized-medicine with advanced stability, based on lipid-based excipients
Abstract:
Statement of the Problem: Lipids and lipid-based excipients are increasingly applied for development of patient-centric products. Their application in the pharmaceutical formulations covers a wide range, from taste-masking of oral dosage forms with modified; both immediate- and extended release profile to development of advanced nanoparticles for pulmonary or parenteral route of drug administration. Despite of this diversity in application, the drug release instability and the lack of mechanistic understanding of it still prevent the larger-scale application of lipidic excipients. This abstract provides a comprehensive overview on the complex solid state behavior of lipids and describes methods for monitoring this behavior for obtaining reliable and reproducible dosage forms.
Methodology & Theoretical Orientation: Solid state behavior of lipids was studied as the response to the composition of formulation and to the critical parameters of the applied product manufacturing process, using X-ray diffraction, PLM and DSC. The applied processes were hot-melt coating for taste-masking and high pressure homogenization for preparation of nanosuspensions. Quality by Design (QbD) tools were used for monitoring the manufacturing process.
Findings: The instability of lipidic formulations can be addressed to both changes in molecular and supra-molecular levels. Changes in molecular level mainly contains polymorphic transformation and alteration in crystallite thickness, which can be monitored by careful selection of formulation composition and process parameters. Certain surfactants can be used as modifier, influencing the kinetic character of polymorphic transition of lipids. Process temperature can be monitored to control both crystallite growth kinetics and polymorphic transition. Understanding the microphase separation of formulations containing emulsifier is necessary and will help to improve the selection of pharmaceutical formulations.
Keynote Forum
Bruno Sarmento
Universidade do Porto, Portugal
Keynote: Nanoparticles-in-vaginal films for combined delivery of anti-HIV microbicide drugs
Time : 12:30-12:50

Biography:
Bruno Sarmento completed his PhD in Pharmaceutical Technology and Degree in Pharmaceutical Sciences at University of Porto, Portugal. He is an Affiliated Researcher at Institute of Investigation and Innovation in Health (i3S) and Institute of Biomedical Engineering (INEB), University of Porto, Portugal. He is an Assistant Professor of Pharmaceutical and Biopharmaceutical Technology at IUCS, Portugal. His current research is focused on “The development of functionalized nanomedicines and their application in the pharmaceutical and biomedical fields; in particular, nano-formulations of biopharmaceutical drugs with interest in diabetes, cancer and infectious diseases”. He has also specialization in “Mucosal tissue engineering models to validate functionalized nanomedicines and to perform in vitro/in vivo correlation”. He has published more than 160 papers in international peer reviewed (ISI) journals, 34 book chapters and more than 180 proceedings. He edited four books, participated in more than 50 invited/selected talks in national and international meetings and was awarded several distinctions. He is a member of Editorial Advisory Board of 10 international journals and has acted as referee for top-ranked journals in his area of expertise and for international funding agencies.
Abstract:
There is an urgent need to reinforce battle against HIV/AIDS, namely by investing more in preventing new infections. Scientific and medical evidence produced over recent years supports that both oral and topical pre-exposure prophylaxis (PrEP) are promising approaches that can reduce sexual transmission of the virus. Still, anti-retroviral with different physicochemical properties may be challenging to combine in one single microbicide product We propose a new system comprising the incorporation of nanoparticles (NPs) into films for the combined vaginal delivery of hydrophobic/hydrophilic molecules. EFV-loaded poly (lactic-co-glycolic acid) NPs were incorporated alongside free TFV into fast disintegrating films during film manufacturing. The delivery system was characterized for physicochemical properties, as well as for genital distribution, local and systemic 24 h pharmacokinetics, and safety upon intra vaginal administration to mice. EFV NPs with diameter of 145 nm were incorporated into a film with TFV. The film was soft and flexible. Disintegration time in simulated vaginal fluid was 9 min, resulting in dispersions with osmolality values near physiologic and pH of 4.24±0.02. The film presented low toxicity to CaSki, HEC-1-A and HeLa cells. NPs were evenly distributed and retained upon vaginal administration to mice. Mild epithelial penetration of NPs was observed. Drug concentrations in vaginal lavages and tissues peaked rapidly after film administration but slowly decreased up to 24 h. Still, drug concentrations were maintained at potentially protective levels. Films were found safe after daily 14 days administration as assessed by histological observation and analysis of IL-1b, IL-6, KC and TNFα levels.
Keynote Forum
Dagmar Fischer
Friedrich-Schiller University, Germany
Keynote: Bacterial nanocellulose as controlled drug delivery system in skin applications
Time : 11:50-12:30

Biography:
Dagmar Fischer is a Pharmacist. She has more than 20 years of experience in the field of “Nanocarriers based on synthetic and natural polymers, their formulation and biopharmaceutical characterization”. Furthermore, she has long-standing successful co-operations with many partners in and outside of Europe, in the field of Nano-safety. After completing her PhD in Habilitation at University of Marburg, she joined a biotech company for several years as Head of Preclinical Research and Development. In 2008, she was appointed as a Professor of Pharmaceutical Technology at University Jena.
Abstract:
The natural hydro-polymer bacterial nano cellulose (BNC) is an innovative biomaterial, produced during fermentation by strains of gram-negative bacteria Komagataeibacter xylinus and consisting of about 1% cellulose and 99% water. Although the chemical formula is identical to plant cellulose, the material favors totally different but outstanding material characteristics due to the three-dimensional network of nano-sized fibers. The interest in BNC as drug delivery system dramatically increased during the last years, as the nano-sized 3D-network of BNC is expected to hold a large amount of drug molecules due to its large surface area. However, the highly hydrophilic character limited a broad application especially for the delivery of lipophilic drugs as well as long-term applications. We developed different loading techniques to accomplish a controlled release of drugs from several hours to weeks using BNC produced under lab-scale as well as under high throughput conditions. Native BNC, hybrid systems with different types of the thermo-responsive block-copolymers poloxamers as well as lipid-modified BNC were established. Depending on the type of modification, not only the drug release profile, but also superior material properties such as high compression stability and water binding could be achieved. Using the antiseptic octenidine as model drug, the antimicrobial activity against Staphylococcus aureus and Pseudomonas aeruginosa was not changed by the use of the modified BNC. Excellent biocompatibility of the loaded BNC could be demonstrated after local administration in a shell-less hen’s egg model. In conclusion, controllable short- and long-term delivery systems consisting of poloxamer and lipid modified BNC could be developed as ready-to-use systems e.g. for dermal wound treatment, cosmetics or the use as implants.
Keynote Forum
Satyanarayana Somavarapu
UCL School of Pharmacy, UK
Keynote: Therapeutic potential against metastatic melanoma of a copper-based aquaporin inhibitor nanoformulated
Time : TBA

Biography:
Somavarapu move to England came with the award of a Commonwealth Fellowship from the Association of Commonwealth Universities and he undertook a PhD at the University of Aston in Birmingham. In 2005 he was appointed as Academic Fellow at The School of Pharmacy and became a lecturer in 2010. He has over hundread publications, including fifty journal articles, over fifty peer-reviewed abstracts and several international conference presentations. He also has six patents on vaccine formulations. His research focus is on designing, understanding & developing technologies for novel nanocarrier systems in overcoming biological barriers for the targeted delivery of small therapeutic molecules and macromolecules (proteins, peptides, siRNA, miRNA) via the pulmonary route in the treatment of lung diseases (lung cancer, asthma, COPD) and ocular conditions.
Abstract:
Fisetin or 3, 3’, 4’, 7-tetrahydroxyflavone is a natural flavonoid which can be found in different fruits and vegetables. Apart from its antioxidant, anti-inflammatory and neuroprotective activities, several studies have shown its anticancer effect in several cancer cell lines (e.g. lung, colon and prostate). This study explores the incorporation of fisetin into the cavity of different cyclodextrins to improve its poor aqueous solubility (< 1 mg/ml), that hinders its delivery. The complex was further engineered into an inhalable dry powder formulation that will potentially be useful to target the lung for therapeutic applications.
The highest complexation was found between fisetin and sulfobutylether-β-cyclodextrin (SBE-β-CD), and addition of 20%v/v ethanol increased the complexation by 5.9-fold. The spray-dried complex from the ethanolic solution showed an improved aerosolization performance, indicated by 2-fold increase in the fine particle fraction, compared to the spray-dried complex from aqueous solution. This may be caused by the lighter and less dense properties of the particles, showed by the pitted morphological surfaces, suggesting a hollower internal structure. Further incorporation of 20%w/w leucine improved the physical and aerosolization properties of the spray-dried complex. The preparation also showed an unchanged cytotoxic activity of fisetin against the human lung adenocarcinoma cell line (A549). In conclusion, the inhalable dry powder of fisetin-SBE-β-CD complex was able to increase aqueous solubility of fisetin and may be useful to deliver fisetin to the deep lung region.
- Peptides and Protein Drug Delivery | Drug Targeting and Design | Nanoparticulate Drug Delivery Systems
Location: London, UK
Session Introduction
Bruno Sarmento
University of Porto, Portugal
Title: Nanoparticles-in-vaginal films for combined delivery of anti-HIV microbicide drugs
Time : 12:30-12:50

Biography:
Bruno Sarmento completed his PhD in Pharmaceutical Technology and Degree in Pharmaceutical Sciences at University of Porto, Portugal. He is an Affiliated Researcher at Institute of Investigation and Innovation in Health (i3S) and Institute of Biomedical Engineering (INEB), University of Porto, Portugal. He is an Assistant Professor of Pharmaceutical and Biopharmaceutical Technology at IUCS, Portugal. His current research is focused on “The development of functionalized nanomedicines and their application in the pharmaceutical and biomedical fields; in particular, nano-formulations of biopharmaceutical drugs with interest in diabetes, cancer and infectious diseases”. He has also specialization in “Mucosal tissue engineering models to validate functionalized nanomedicines and to perform in vitro/in vivo correlation”. He has published more than 160 papers in international peer reviewed (ISI) journals, 34 book chapters and more than 180 proceedings. He edited four books, participated in more than 50 invited/selected talks in national and international meetings and was awarded several distinctions. He is a member of Editorial Advisory Board of 10 international journals and has acted as referee for top-ranked journals in his area of expertise and for international funding agencies.
Abstract:
Statement of the Problem: There is an urgent need to reinforce battle against HIV/AIDS, namely by investing more in preventing new infections. Scientific and medical evidence produced over recent years supports that both oral and topical pre-exposure prophylaxis (PrEP) are promising approaches that can reduce sexual transmission of the virus. Still, anti-retroviral with different physicochemical properties may be challenging to combine in one single microbicide product.
Methodology & Theoretical Orientation: We propose a new system comprising the incorporation of nanoparticles (NPs) into films for the combined vaginal delivery of hydrophobic/hydrophilic molecules. EFV-loaded poly (lactic-co-glycolic acid) NPs were incorporated alongside free TFV into fast disintegrating films during film manufacturing. The delivery system was characterized for physicochemical properties, as well as for genital distribution, local and systemic 24 h pharmacokinetics, and safety upon intra vaginal administration to mice.
Results: EFV NPs with diameter of 145 nm were incorporated into a film with TFV. The film was soft and flexible. Disintegration time in simulated vaginal fluid was 9 min, resulting in dispersions with osmolality values near physiologic and pH of 4.24±0.02. The film presented low toxicity to CaSki, HEC-1-A and HeLa cells. NPs were evenly distributed and retained upon vaginal administration to mice. Mild epithelial penetration of NPs was observed. Drug concentrations in vaginal lavages and tissues peaked rapidly after film administration but slowly decreased up to 24 h. Still, drug concentrations were maintained at potentially protective levels. Films were found safe after daily 14 days administration as assessed by histological observation and analysis of IL-1b, IL-6, KC and TNFα levels.
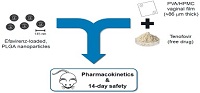


Conclusion & Significance: The proposed NPs-in-vaginal film is a promising new system that can adequately combine microbicide drugs with different solubility profiles. Results support that the system may be safe and able to provide sustained and potentially effective drug levels for preventing vaginal HIV transmission.
Imran Saleem
Liverpool John Moores University, UK
Title: Dry powder inhalation of Pneumococcal protein-based nanocarrier vaccine
Time : 12:50-13:10

Biography:
Imran Saleem is a Reader in Nanomedicine within the School of Pharmacy & Biomolecular Sciences, Liverpool John Moores University, UK. He has been working in and has published numerous papers in the area of pulmonary drug delivery since 2006. His research is aimed at developing novel delivery systems for targeting therapeutic agents to their site of action, with particular emphasis on lung diseases via dry powder pulmonary delivery. He has over 10 years’ experience in the area of nanoparticle formulation and drug delivery systems, and has published extensively in peer-reviewed journals, conference abstracts and book chapters.
Abstract:
There is a huge drive in the vaccine research field, pharmaceutical industry and Bill Gates Foundation for effective targeting of dendritic cells (DCs) to enhance the immune response and for needle-free vaccination. The aim of this study was to adsorb pneumococcal protein (PspA), onto poly(glycerol adipate-co-ω-pentadecalactone), PGA-co-PDL, nanoparticles (NPs) to target lung DCs. Further to formulate these NPs into dry powder nanocomposite microparticles (NCMPs) suitable for pulmonary vaccine delivery. NPs were prepared using an emulsion solvent evaporation method and PspA was adsorbed onto the surface of NPs (100: 20 [NP: PspA]). The NPs were spray-dried in an aqueous suspension of leucine (1:1.5) to produce NCMPs and characterised in terms of particle size, loading, cell viability, protein stability (SDS-PAGE), integrity (circular dichroism, CD), antigenicity (ELISA), immunization and aerosolisation studies. The NPs produced were 322.83 ± 4.25 nm in size with PspA loading 19.68 ± 2.74 µg/mg. The NCMPs resulted in a fine particle fraction (FPF%) >75%. The NPs appear to be well tolerated by DCs cell lines ≥90% cell viability) at 19.5µg/mL after 4h exposure. SDS-PAGE, CD (α-helical decreased < 13% vs standard PspA) and the antigenicity (>95%) confirmed that PspA was stable in both formulations after spray-drying. The cfu in BALF of mice challenged with pneumococcal bacteria was signifcantly less compared to PspA alone in the lungs or via subcutaneous injection. The PspA loaded NPs were incorporated into NCMPs having excellent aerosolisation characteristics whilst maintaining protein activity. Hence, it may be feasible to use these carriers for pulmonary vaccine delivery.
Marta Truffi
University of Milano, Italy
Title: Targeting active bowel inflammation foci by MAdCAM-1-specific nanoparticles
Time : 14:00-14:20

Biography:
Marta Truffi has her expertise in nano-biotechnology and cellular biology. Her current research is focused on the study of targeted nanosystems that will provide specific diagnosis and therapy of inflammatory bowel diseases. Her work involves in-depth study of the pathogenesis and identification of novel therapeutic targets, investigation of functional interactions between nanoparticles and cell cultures/tissues, development of in vitro and in vivo experimental models of human diseases, study of nanoparticles biodistribution and systemic toxicity. She is also interested in nanoparticles for breast cancer treatment, by coupling targeted delivery of chemotherapeutics with modulation of cancer-associated miRNAs, in order to improve patients’ responsiveness to therapy.
Abstract:
Statement of the Problem: Currently, the evaluation and treatment of inflammatory bowel disease (IBD) commonly relies on aspecific clinical signs of bowel inflammation, while specific targeted devices are still lacking. Mucosal addressin cell-adhesion molecule-1 (MAdCAM-1) has been proposed as a marker of bowel inflammation. It is upregulated on gut endothelium in IBD and is finely related to IBD activity and response to therapy. Here, we investigate a smart nano-platform targeted toward MAdCAM-1 for site-specific nano-theranostics in a preclinical model of IBD.
Methodology & Theoretical Orientation: We coupled anti-MAdCAM-1 antibodies to the surface of manganese oxide nanoparticles, and analyzed nanoconjugate biodistribution and safety in a murine model of IBD, by intravenous injection at the time of early acute phase of the disease.
Findings: Manganese oxide nanoparticles revealed good stability and negligible toxicity toward endothelial cell culture. Twenty-four hours post intravenous administration in colitic mice, fluorescent anti-MAdCAM-1-nanoparticles localized in the inflamed bowel, and specifically accumulated in the proximal part of the colon. By contrast, untargeted nanoparticles were more rapidly washed out. Nanoparticles did not induce histologic lesions in non-target organs.
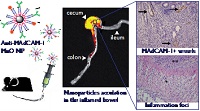
Conclusion & Significance: Anti-MAdCAM-1-nanoparticles uncovered active bowel inflammation foci, by following the expression pattern of MAdCAM-1 on mucosal vessels. The implementation of this nano-platform for early and specific theranostics applications appears promising for refining clinical care and management of IBD.
Satyanarayana Somavarapu
UCL School of Pharmacy, UK
Title: Formulation of inhaled phytochemicals
Time : 14:20-14:40

Biography:
Satyanarayana Somavarapu move to England came with the award of a Commonwealth Fellowship from the Association of Commonwealth Universities and he undertook a PhD at the University of Aston in Birmingham. In 2005 he was appointed as Academic Fellow at The School of Pharmacy and became a lecturer in 2010. He has over hundread publications, including fifty journal articles, over fifty peer-reviewed abstracts and several international conference presentations. He also has six patents on vaccine formulations. His research focus is on designing, understanding & developing technologies for novel nanocarrier systems in overcoming biological barriers for the targeted delivery of small therapeutic molecules and macromolecules (proteins, peptides, siRNA, miRNA) via the pulmonary route in the treatment of lung diseases (lung cancer, asthma, COPD) and ocular conditions.
Abstract:
Fisetin or 3, 3’, 4’, 7-tetrahydroxyflavone is a natural flavonoid which can be found in different fruits and vegetables. Apart from its antioxidant, anti-inflammatory and neuroprotective activities, several studies have shown its anticancer effect in several cancer cell lines (e.g. lung, colon and prostate). This study explores the incorporation of fisetin into the cavity of different cyclodextrins to improve its poor aqueous solubility (< 1 mg/ml), that hinders its delivery. The complex was further engineered into an inhalable dry powder formulation that will potentially be useful to target the lung for therapeutic applications.
The highest complexation was found between fisetin and sulfobutylether-β-cyclodextrin (SBE-β-CD), and addition of 20%v/v ethanol increased the complexation by 5.9-fold. The spray-dried complex from the ethanolic solution showed an improved aerosolization performance, indicated by 2-fold increase in the fine particle fraction, compared to the spray-dried complex from aqueous solution. This may be caused by the lighter and less dense properties of the particles, showed by the pitted morphological surfaces, suggesting a hollower internal structure. Further incorporation of 20%w/w leucine improved the physical and aerosolization properties of the spray-dried complex. The preparation also showed an unchanged cytotoxic activity of fisetin against the human lung adenocarcinoma cell line (A549). In conclusion, the inhalable dry powder of fisetin-SBE-β-CD complex was able to increase aqueous solubility of fisetin and may be useful to deliver fisetin to the deep lung region.
Khuloud Al Jamal
King’s College London, UK
Title: Double-targeting of glioma in mice using LRP1-targeting carbon nano-needles
Time : 14:40-15:00

Biography:
Khuloud T. Al-Jamal is a Chair of Drug Delivery & Nanomedicine at King’s College London (KCL). She was awarded the Overseas Research Award Scheme Scholarship from The University of London (2000-2004) to complete her PhD in Drug Delivery from The School of Pharmacy (currently known as UCL-School of Pharmacy). She was awarded the prestigious CW Maplethorpe Research and Teaching Postdoctoral Fellowship from The University of London (2005-2007) and started her academic career as a lecturer at KCL in 2011. She was awarded the prestigious Royal Pharmaceutical Society Science Award in 2012 in recognition for her outstanding scientific achievements in the field of Nanomedicine. She has developed an extensive experience in designing and developing novel nanoscale delivery systems including dendrimers, liposomes, quantum dots, polymers, viral vectors, chemically functionalised carbon nanotubes and graphene oxide. Her current work involves pre-clinical translation of novel nanomaterials designed specifically for drug, protein, nucleic acids and radionuclide delivery for therapeutic or diagnostic applications.
Abstract:
Statement of the Problem: Brain disorders are on the rise accounting for almost 12% of world mortalities every year. Despite extensive research in drug development, brain disorders are still largely untreated due to the inability to deliver current therapeutics to the brain across the BBB. Chemically functionalized carbon nanotubes (f-CNT) constitute a novel class of nanomaterials with attractive physical, chemical and electronic properties. One interesting characteristic of f-CNTs is their ability to translocate across plasma membranes and enter the cells either passively by direct translocation across membranes or actively via endocytosis. In this study, the brain uptake properties of multiwalled f-CNTs (f-MWNTs) were studied in in vitro and in vivo.
Methodology: An in vitro model consisting of PBEC and astrocytes were co-cultured in a Transwell™ system. Percentage of BBB crossing of radiolabelled [111In] DTPA-MWNTs was assessed at 37 °C up to 72 h or with an initial incubation at 4 °C for 4 h. Ultrathin sections of PBEC were imaged using electron microscopy. Brain uptake in vivo was evaluated by SPECT/CT imaging and gamma counting following intravenous injection of [111In] DTPA-MWNTs in mice.
Findings: The percentage transport of [111In] DTPA-MWNTs across PBEC (Figure A) increased over the course of 72 h. The initial 4 h-incubation at 4 â—¦C resulted in a slight but significantly lower % transport than that obtained at 37 â—¦C (P= 0.0005). This difference was abolished upon the re-incubation at 37 â—¦C at 72 h. The penetration process was captured by electron microscopy. The accumulation in mouse brain was confirmed by SPECT/CT imaging (Figure B). Superior brain uptake of ~2-5% ID/g was measured by gamma counting after whole body perfusion.
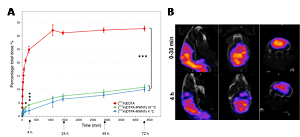
Conclusion & Significance: This is the first evidence of f-MWNTs translocation across the BBB. The significant reduction in BBB crossing at 4 °C confirmed the uptake was driven by an energy-dependent pathway. Electron micrographs revealed transcytosis of f-MWNTs and its sequence as a function of time. f-MWNT’s are able to access mice brain after i.v. injection.
Dimitrios A Lamprou
University of Kent, UK
Title: Metrology in pharmaceutical manufacturing
Time : 15:00-15:20

Biography:
Dimitrios Lamprou (Beng, PgCert, PgDip, MSc, PhD, MBA) is Associate Professor in Pharmaceutics appointed in September 2016 at University of Kent, and has been trained in multidisciplinary areas and worked in first class laboratories. Dr Lamprou has experience of teaching in Higher Education, conducting research and securing National and International funding (over £1M). Dr Lamprou has authored over 40 articles in high impact multidisciplinary journals, and over 130 poster and podium presentations at national and international conferences, that includes over 40 invited talks, and has experience of supervising Postdoc's, 20+ Ph.D students and 70+ Masters level research projects.
Abstract:
Structure-property relationships are often poorly defined in advanced continuous pharmaceutical manufacturing processes and products and hence it is difficult to control final product performance to the required degree to deliver advanced functionality. The dynamics of particles within complex mixtures and the effect of processes and storage on their disposition and microstructure is also challenging to measure. Hence, there is a clear need to have techniques for analysis and measurement of composition, dynamics and structure with increased spatial and temporal resolutions. Secondary Ion Mass Spectrometry (SIMS) is a technique that enables the analysis of the ionized particles (secondary ions) emitted when a surface is bombarded by energetic species (primary ions). To apply this technology to the study of Pharmaceutical Products is extremely interesting in order to investigate the precise distribution of Active Pharmaceutical Ingredients (APIs), impurities or excipients in the formulated product, also in a three dimensional visualization, to have a better understanding of the impact that this could have on its final performance. The aim of this work was to visualize the lateral and depth distribution of the drug in the polymer matrix, using ToF-SIMS, and to relate this data to the drug elution rate. The funduings was supported with data from other techniques, such as Atomic Force Microscopy (AFM), Raman Conmfocal Imagine, and microCT.
Wafa T. Al-Jamal
University of East Anglia, UK
Title: Targeted drug delivery to enhance cancer therapy and reduce its side effects
Time : 15:20-15:40

Biography:
Wafa Al-Jamal is an overseas and a UK-registered pharmacist. She completed her PhD in Drug Delivery in 2008 at UCL School of Pharmacy, London. She is currently a Prostate Cancer Research Fellow at The School of Pharmacy, University of East Anglia (UEA). She joined UEA as a Lecturer in Drug Delivery and Nanomedicine in 2013, after working as a senior research fellow at University College London and King’s College London. She was the GSK Emerging Scientist Award winner for 2015. Her main research interest focuses on engineering novel nanomaterials for biomedical applications. Her research has been funded by the Royal Society, Prostate Cancer UK, Research Council (EPSRC). She has published over 35 papers in high impact journals. Currently, she is a member the PCUK Research Advisory Committee and Visiting Professor at Guizhou Medical School, China.
Abstract:
Most cancer chemotherapeutics lack tissue specificity, resulting in many undesirable side effects. Delivering drugs selectively to the tumour tissues could ultimately increase local drug concentrations at the tumor without the need to escalate the administrated doses in patients. A wide range of drug delivery systems has been developed to alter the pharmacokinetics of the drug molecules and enhance their tumour targeting. Furthermore, several approaches have been explored to increase the bioavailability of drugs at the site of action, utilizing the unique characteristics of the tumor microenvironment, such as overexpressed enzymes, acidic pH, and hypoxia, or using external triggers, such as heat, ultrasound, and light. In this talk will describe the latest delivery systems that we have developed in our laboratory to enhance the tumour accumulation of anticancer drugs, utilising internal and external triggers.
Sharif Abdelghany
University of Jordan, Jordan
Title: PLGA nanoparticles entrapping amikacin and moxifloxacin as a potential host-directed agent therapy for multidrug resistant tuberculosis
Time : 15:40-16:00

Biography:
Sharif Abdelghany completed his PhD from Queen’s University Belfast, UK in drug delivery and Biomaterials in 2012. During his PhD, he developed a wide expertise in the formulation of targeted polymeric nanoparticles for cancer treatment and infectious diseases. After that, He joined the university of Jordan as an assistant professor in the department of Pharmaceutics and Pharmaceutical Technology where he developed polymeric nanoparticles formulation for the treatment of tuberculosis. He also received an endeavor research fellowship to conduct a short term fellowship for the dual nanoparticles entrapment of second line anti-TB drugs at the university of Sydney, Australia (April 2015-August 2015).
Abstract:
Polymeric nanoparticles have been widely investigated as a controlled release drug delivery platform for the treatment of tuberculosis (TB). These nanoparticles were also readily internalised into macrophages, leading to high intracellular drug concentration. In this study two anti-TB drugs, amikacin and moxifloxacin were encapsulated into PLGA nanoparticles. The novelty of this work appears in: (1) the efficient encapsulation of two hydrophilic second-line anti-TB drugs,and (2) intramacrophage delivery of this synergistic combination potentially for rapid treatment of multi-drug resistant TB (MDR-TB). Two water-oil-water (w/o/w) emulsion strategies were employed in this study: (1) alginate coated PLGA nanoparticles, and (2) alginate entrapped PLGA nanoparticles. The average particle size and polydispersity index (PDI) of the alginate coated PLGA nanoparticles were found to be unfavourably high with values of 640 ± 32 nm and 0.63 ± 0.09, respectively. In contrast, the alginate entrapped PLGA nanoparticles were within the desirable particle size range of 282 - 315 nm and the PDI was 0.08 - 0.16, and therefore were chosen for subsequent studies. Alginate entrapped PLGA nanoparticles yielded a drug loading of over 10 µg/mg powder for amikacin, and more than 5 µg/mg for moxifloxacin and entrapment efficiencies range of approximately 25-31% for moxifloxacin and 51-59% for amikacin. To study macrophage uptake efficiency, the nanoparticles of alginate entrapped nanoparticle formulation were loaded with acridine orange as a marker, seeded to THP-1 derived macrophages and viewed under confocal microscopy. The particles were readily internalised into the macrophages and highly concentrated in the nucleus region. Furthermore, the anti-mycobacterial activity of the drug-loaded particles was evaluated using M. tuberculosis-infected macrophages, which revealed a significant reduction (4 log reduction) of viable bacterial count compared to the untreated group. In conclusion, the amikacin-moxifloxacin alginate entrapped PLGA nanoparticles are promising for further in vivo studies. Particle internalisation of acridine orange loaded PLGA nanoparticles by THP-1 derived macrophages using confocal microscopy. The cells were treated with acridine orange loaded nanoparticles at two different concentrations of: (A) 100 µg/mL and (B) 10 µg/mL, and (C-D) PBS cells. (E) The fluorescence intensity of acridine orange loaded nanoparticles and DAPI with increasing Z dimension (corresponds to the intensity in different layers of the macrophage cell). The green color corresponds to the distribution of nanoparticles in the cytosol of THP-1 cells. The blue colour corresponds to the DAPI stained nucleus.
Poster Presentations/Exhibitions/Networking/B2B Meetings
Poster Presentations/Exhibitions/Networking/B2B Meetings
Title: Poster Presentations/Exhibitions/Networking/B2B Meetings
Biography:
Poster Sessions will be conducted in the Foyer Area...
Abstract:
For more details, PS: http://novel-drugdelivery-systems.pharmaceuticalconferences.com/poster-presentation.php
