Day 3 :
Keynote Forum
Olivia Merkel
Ludwig-Maximilians-Universität München, Germany
Keynote: In-Situ Forming Gel Devices as Local Depot Therapeutic for Rheumatoide Arthritis
Time : 09:30-10:10

Biography:
Olivia Merkel, is a Professor of Drug Delivery in the Department of Pharmacy at LMU Munich. From 2011 until 2016 she was an Assistant Professor of Pharmaceutics and an Associate Faculty Member of Oncology at Wayne State University and Barbara Ann Karmanos Cancer Institute in Detroit. She became a Registered Pharmacist in 2005. In 2006, she received a MS in Pharmaceutics from Martin-Luther-Universität Halle-Wittenberg, and a PhD in Pharmaceutics from Philipps-Universität Marburg in 2009. She received several awards, including an ERC Starting Grant, the Galenus Foundation Technology Award, the Wayne State College of Pharmacy Young Investigator Award, the European Federation for Pharmaceutical Science Young Pharmaceutical Investigator Award, an invitation to the Lindau Nobel Laureates Meeting, the Carl-Wilhelm-Scheele-Award by the German Pharmaceutical Society (DPhG) and the award for the best PhD thesis at Philipps-Universität Marburg. Currently Prof. Merkel’s research focuses on targeted siRNA delivery in cancer and inflammatory diseases
Abstract:
Statement of the Problem: More efficient anti-inflammatory therapies with reduced side effects are needed to treat Rheumatoid arthritis (RA), a chronic and disabling autoimmune condition that affects about 1% of the population in developed countries. Even though a multitude of cell types is involved, macrophages play a central role in the pathophysiology of RA. Locally implantable, targeted, macrophage-specific RNA interference (RNAi)-based therapies could therefore revolutionize RA therapy.
Methodology: Three-layered micelles (3LM) encapsulating nucleic acids were formed from triblock copolymers of PLLA-PEI-PLLA and PLLA-PEG-PLLA in a three-step procedure. Their structure and DNA entrapment in the core was determined by TEM. Hydrodynamic diameters and zeta potentials were measured by dynamic light scattering. DNA release in neutral and acidic pH was detected by modified SYBR Gold assays. For targeting of activated macrophages, folic acid (FA) was attached to the PEG-chain of a PLLA-PEG diblock affording PLLA-PEG-FA. Subsequently, 3LM were formed with PLLA-PEG-FA in the outer polymer shell. RAW264.7 cells were activated with LPS or left resting. Primary macrophages were isolated after in vivo activation. One day later, the cells were transfected with targeted and non-targeted 3LM, and GFP expression was quantified by flow cytometry. Thermoresponsive hydrogels were obtained by stereocomplexing 3LM which contain PLLA-PEG-PLLA in the outer core with PDLA-PEG-PDLA.
Conclusion & Significance: The core-corona structure and efficient DNA entrapment in the core were confirmed by TEM. Sizes were found to be less than 200 nm, and the encapsulation efficiency of DNA was optimized. 3LM were stable at neutral pH but released DNA in an acidic environment. 3LM were efficiently targeted to activated macrophages by blending PLLA-PEG-FA in the outer layer, while non-targeted micelles or PEI polyplexes were not efficiently taken up. Stereocomplexes of 3LM formed hydrogels above their phase transition temperature and released 3LM in acidic environment that efficiently transfected primary macrophages.
- Young Researchers Forum
Location: London, UK

Chair
Olivia Merkel
LMU Munchen, Germany

Co-Chair
Imran Saleem
Liverpool John Moores University, UK
Session Introduction
Marco Contardi
Italian Institute of Technology, Italy
Title: Transparent ciprofloxacin-polyvinylpyrrolidone antibiotic films and nanofiber mats as potential skin and wound care dressings
Time : 10:10-10:25

Biography:
Marco Contardi is a PhD student of Smart Materials group of the Nanophysics Department of Italian Institute of Technology in Genova. He obtained a Master’s degree in pharmaceutical chemistry and technology at the University of Palermo. After the graduation he began an internship period between Institute of Biophysics at National Council of Research of Palermo and Department of Physics and Chemistry of Palermo. Recently, he published 3 articles as co-author and his contribute regarded hydrogels preparation and their rheological and biological characterization and spectrofluorometric analysis of metformin.
Abstract:
Wound dressings have been evolving steadily in close conjunction with recent trends and advances in bio-based polymer processing. Dressings grew from materials or tapes that simply covered and concealed the wound, to materials that can interact with wounds so that important wound healing factors such as moisture management, active ingredient delivery and interaction with cells or proteins can be tuned or enhanced in situ. For chronic non-healing wounds or burns, for instance, one of the most important aspects is to prevent infection. As such, dressings should provide a continuous or quick release of the antiseptic agent at the wound surface to provide a long-lasting antimicrobial action in combination with maintenance of physiologically moist environment for healing. Most commercially available wound dressings are frequently opaque. Visual observation of the healing process is therefore prevented. Infection or other complications cannot be detected with opaque coverings. During inspection, dressing removal causes discomfort but also healing disruption, bleeding and granuloma formation. Transparent wound dressings are therefore highly desirable. Particularly, antiseptic loaded and adhesive free transparent wound dressings that can be easily absorbed by the wound are highly desirable. Hence, we demonstrate a facile water-based solvent casting process to incorporate a common insoluble antibiotic known as ciprofloxacin (Cipro) in its unmodified form in polyvinylpyrrolidone polymer using aqueous acetic acid solutions. Acetic acid enabled transparency, enhancement in antiseptic effect and softer films. Preliminary in vivo tests on C57BL/6J mice displayed good capacity to absorb exudate and dissolution rate of transparent antibiotic films on a model wound.
Fatma Demir Duman
Koc University, Turkey
Title: Development of cationic Ag2S NIR emitting QD’s as new generation of theranostic nanoparticles
Time : 10:25-10:40

Biography:
Fatma Demir Duman has her expertise in design, synthesis and characterization of near-IR emitting quantum dots and gold nanoparticles and their applications as optical imaging, gene and drug delivery agents and investigation of in vitro and in vivo effects of designed nanostructures.
Abstract:
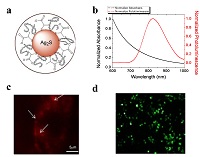
Quantum dots are semiconducting nanocrystals with diameters between 2-10 nanometers. Strong signal brightness, resistance to photo bleaching, size-tunable emission and large absorption coefficient across a wide spectral range make them powerful agent against conventional organic fluorescence dyes used in bioimaging applications. In addition to their optical imaging ability, quantum dots have multifunctional properties such as functionalization with targeting ligands for site specific delivery and loading with therapeutic drugs, peptides or oligonucleotides for drug and gene delivery applications thanks to their high surface to volume ratio. However, most of the synthesized QD’s emit in the visible region and they contain heavy metals such as Cd, Pb or Hg, which have intrinsic toxicity to living organisms. Ag2S QDs developed in recent years are of great interest with their excellent biocompatibility and strong emission intensity in near infrared region (NIR) of optical spectrum, offering higher photon penetration depth, lower absorption and scattering of light by cellular components and lower auto-fluorescence in the living tissue compared to visible region. Yet, the Ag2S compositions reported are still limited to anionic and PEGylated ones. We have focused on the development of first cationic Ag2S QDs for combined action of optical imaging and gene therapy. Here, we will discuss the synthesis of
- Nanotechnology in Drug Delivery | Pharmaceutical Nanotechnology | Biomaterials in Drug Delivery
Location: London, UK
Session Introduction
António José Ribeiro
University of Coimbra, Portugal
Title: Nanoparticles for oral delivery of Insulin: Facts and safety concerns
Time : 11:00-11:20

Biography:
Antonio Ribeiro is a Professor of Pharmaceutical Technology at the Faculty of Pharmacy of University of Coimbra where he managed a high international reputed research group. He has a Ph.D Degree in Pharmaceutical Development and Biopharmacy and his research has been focused on design of delivery systems for peptidic and protein drugs. He has published more than sixty peer-reviewed publications, among which several very highly cited, and he has been a keynote speaker and presented various talks all over the world. He is also an expert and consultant in intellectual property of drugs for pharmaceutical industry. He serves as an Editorial member of several publications and as a consultant for several research agencies mostly related to Diabetes and Nanotechnology.
Abstract:
Nanotechnology-based approaches towards an oral delivery of macromolecules such as insulin are increasingly therapeutic approaches towards the prevention or treatment of diabetes. Nanoencapsulation of insulin increases its protection against enzymatic degradation, and facilitates its absorption through intestinal membranes. However, the intestinal absorption of insulin nanoparticles may be related to a stimulatory reaction induced by the local mucosa immune system. Intestinal epithelium is an immune privileged organ capable of mediating immune reactions either playing a local protective role or by triggering an inflammatory response to the presence of nanoparticles. Compromising safety of insulin delivered by nanoparticles, by inadequacy or insufficiency of studies, may lead to exacerbation of the inflammatory pathways conducive to unwanted local and other severe adverse effects. Therefore, it is imperative to include comprehensive safety assays in early pre-clinical studies in order to increase the representativeness of the results and strengthen the potential of oral delivery of insulin by means of nanoparticles. Herein, focus will be put on recent reports in oral delivery of insulin nanoparticles for diabetes as well a critical analysis of the safety studies supporting their pre-clinical development. The improvement of early safety assessment by transitioning to quantitative, nanoparticle composition-immune performance relationship studies in representative models will also be elucidated. Thereby, the role and importance of rational optimization in the development of “safe by design” insulin nanoparticles will be contextualized in the field of diabetes..
Maria Manuela Gasper
University of Lisboa, Portugal
Title: Therapeutic potential against metastatic melanoma of a copper-based aquaporin inhibitor nanoformulated
Time : 11:20-11:40

Biography:
Maria Manuela Gaspar has completed her PhD in 2005 in Pharmaceutical Technology at University of Lisbon and Post-doctoral studies at University of Dublin, Trinity College. She is a Researcher at Research Institute for Medicines, iMed.ULisboa, University of Lisbon. The area of research has been focused on “Design, development and biological evaluation of drug delivery systems for improving the therapeutic index of incorporated molecules in infectious, inflammatory and cancer animal models”. She is a Co-author of numerous patents, papers in peer-reviewed journals and book chapters.
Abstract:
Statement of Problem: Clinical and preclinical studies evidence the overexpression of aquaporin (AQPs) in a high number of cancers. In this sense, the development of selective AQP inhibitors represents an alternative therapeutic strategy for these pathologies. Metallodrugs of copper and gold are potential candidates to fulfill this need. However, to promote a preferential target to tumor sites in vivo, the design and development of novel technologies based on nanostructured materials such as liposomes acting mainly as vectors for metallodrugs delivery represents a stimulating research area. Cuphen, a potent copper-based aquaporin inhibitor, was selected as metallodrug in the present work to be nanoformulated in liposomes.
Methodology: Cuphen was incorporated in liposomes by the dehydration-rehydration method. Cuphen liposomes were characterized in terms of mean size, poly dispersion index, zeta potential and encapsulation parameters using different lipid compositions. The ability of Cuphen to induce cancer cell death was evaluated by MTS and ViaCount assays against several human and mouse cell lines (A431, MNT-1, HaCaT, B16F10 and C26). The hemolytic activity of Cuphen in free and liposomal forms using EDTA-preserved peripheral human blood from voluntary donors was carried out. In vivo toxicity studies were performed in healthy mice and the therapeutic effect was evaluated in a murine melanoma xenograft model.
Findings: In vitro studies illustrated the anti-proliferative effects of Cuphen in different cancer cell lines. In vivo studies revealed no toxic effects after parenteral administration in healthy mice. A higher anti-cancer effect in a murine melanoma xenograft model in terms of survival rate was observed for the metallodrug nanoformulated in long circulating liposomes.
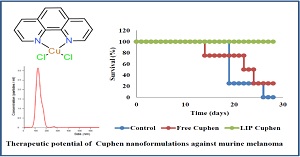
Conclusion: Cuphen liposomes are considered as a very attractive nano-formulation with therapeutic potential against melanoma due to their preferential extravasation and accumulation in solid tumors.
Anselmo J. Otero-Gonzalez
Havana University, Cuba
Title: Cm-p5: an antifungal mollusc-derived peptide conjugated with citric acid-modified manganese-ferrite nanoparticles with enhanced activity against Candida albicans in vitro
Time : 11:40-12:00

Biography:
Anselmo J. Otero-Gonzalez, Microbiologist, Havana University, 1978, PhD, National Centre for Scientific Research, Havana, 1987, Doctor in Science, Havana University, 2008, now is Senior Researcher, Antimicrobial Peptide lab, Faculty of Biology, Havana University, Cuba and president of National Board for PhD examination in Cuba. He is professor of Cell Engineering and Immunology. 1981: awarded with a fellowship for attending the Uppsala Separation School, Biomedical Centre University of Uppsala, Sweden. 1983: doctoral stay at the Department of Genetics, Pennsylvania University, Philadelphia, USA for monoclonal antibody generation. 1991: post-doctoral stay regarding Cell banking at the European Collection of Animal Cell Cultures, Porton Down, Salisbury. 1992: fellowship for a research project (AIDS-HIV vaccine), Swedish Centre of Disease Control, Stockholm, Sweden. 2000: awarded with a postdoctoral fellowship, David Rockefeller Centre of Latin-American Studies, Harvard School of Public Health, Harvard University, Boston, USA. 2011-12: post-doctoral stay at Harvard Medical School for characterizing the antifungal peptide Cm-p5. He also has (2008-present) collaboration with the Bioorganic Department, Leibniz Institute for Plant Biochemistry, Halle (Saale), Germany regarding antimicrobial peptide isolation, evaluation and characterization. Dr. Otero has more than 90 scientific articles in recognized journals and more that 145 abstract and presentations in scientific events.
Abstract:
Antimicrobial peptides form part of the first line of defence against pathogens for many organisms. Current treatments for fungal infections are limited by drug toxicity and pathogen resistance. Cm-p5 (SRSELIVHQRLF), a peptide derived from the marine mollusc Cenchritis muricatus (Gastropoda: Littorinidae) has a significantly increased fungistatic activity against pathogenic Candida albicans, exhibiting low toxic effects against a cultured mammalian cell line. Cm-p5 as characterized by Polarimetry of Circular Dichroism and Spectroscopy of Nuclear Magnetic Resonance. The antimicrobial activity of different types of nanoparticles has been previously demonstrated. Specifically, magnetic nanoparticles have been widely studied in biomedicine due to their physicochemical properties. The citric acid-modified manganese ferrite nanoparticles used in this study were characterized by high-resolution transmission electron microscopy, which confirmed the formation of nanocrystals of approximately 5 nm diameter. These nanoparticles were able to inhibit Candida albicans growth in vitro. However, the nanoparticles were not capable of inhibiting Gram-negative bacteria Escherichia coli or Gram-positive bacteria Staphylococcus aureus. The antifungal peptide Cm-p5 was conjugated to the modified manganese ferrite nanoparticles. The antifungal activity of the conjugated nanoparticles was higher than their bulk counterparts. This conjugate proved to be nontoxic to a macrophage cell line at concentrations that showed antimicrobial activity.
Pranav Shah
Uka Tarsadia University, India
Title: Asenapine Maleate loaded solid lipid nanoparticles for oral delivery
Time : 12:00-12:20

Biography:
Pranav Shah has completed his PhD from Maharaja Sayajirao University of Baroda, India. He is an Associate Professor at Maliba Pharmacy College, Gujarat, India. He has published 16 papers in reputed journals, two book chapters and one book. He has presented several papers in national and international conferences.
Abstract:
Asenapine maleate (ASM) is a new second-generation antipsychotic approved in August 2009 by U.S FDA for the acute treatment of schizophrenia and manic or mixed episodes associated with bipolar disorder in adults. It shows poor oral bioavailability of <2% due to extensive first pass metabolism in liver. The present study was aimed at developing and characterizing solid lipid nanoparticles (SLNs) of ASM. SLNs were prepared by solvent injection method by employing compritol ATO 888 as the lipid matrix and poloxamer 188 as stabilizer. A 32 full factorial design was employed to study the influence of independent variables (amount of lipid and % surfactant) on dependent variables (particle size, and % entrapment efficiency). Optimized ASM-loaded SLNs were further studied for zeta potential, in vitro drug release and TEM. Nanoparticles were lyophilized to improve the physical stability and obtain free flowing powder. Mannitol was employed as a cryoprotectant. Lyophilized ASM-loaded SLNs were characterized using DSC and XRD. The optimized ASM-loaded SLNs exhibited mean particle size 318.5±3.2 nm; polydispersity index of 0.255; zeta potential -29.75±(-0.92) mV; entrapment efficiency 53.13±1.77%; drug release extended up to 36 hours. TEM image exhibited spherical smooth surfaced nanoparticles. Accelerated stability studies of optimized ASM-loaded SLNs and lyophilized ASM-loaded SLNs revealed its stability. The developed formulation holds promising future due to reduction in dose and dosing frequency and thus reduces dose related side effects and improved patient compliance.
Muhammad Hanif
Bahauddin Zakariya University, Pakistan
Title: Thiolated pectin conatining hydrogels for controlled drug delivery of citrizine hydrochloride with its in-vitro studies
Time : 12:20-12:40

Biography:
Muhammad Hanif has completed his PhD from University of Karachi, Pakistan. He is the Managing Editor of Pakistan Journal of Pharmaceutical Research, a biannually published journal. He has published more than 45 papers in reputed journals and has supervised 15 MPhil and six PhD students. His speciality areas are “Formulation development of controlled release dosage forms, micro-encapsulations, hydrogels, in vitro and in vivo correlations (IVIVC), pharmacokinetic and stability studies.
Abstract:
Use of pectin in controlled release dosage form is reported in the previous literature and many dosage forms like tablets, microspheres and hydrogels were developed. Some studies reported the mucoadhesive properties of the pectin when used as drug career. In our study, we have developed thiolated pectin first by adding the thiol containing substances and then converted it into the hydrogel, having the capacity to use both lipophilic and hydrophilic drugs. Central composite rotatable design was successfully applied with the three variables and nine hydrogel formulations were planned in such a way that each three contains the difference in concentration of polymer, monomer and cross linking agent. Prepared formulations were further subjected to swelling, porosity, sol-gel fraction, cross-linked density and drug loading studies. Drug release studies were performed in USP 2 dissolution apparatus having the volume 900 ml at 370C and with 100 rpm rotation. In vitro kinetic model dependent and model independent approaches were applied to select the one best hydrogel formulation. Morphological studies like SEM, FTIR, TGA, DSC and XRD were performed to check any interaction between the polymer and drug. Prepared hydrogels were dried at room temperature first and then in oven at 450C. Maximum swelling observed was pH dependent and at 7.5 pH all formulations showed more than 100% swelling due to the presence of carboxylic acid group in the acrylic acid which has the pKa value four. Mucoadhesion in the thiolated pectin was increased as we increased the concentration of thiol glycolic acid. More than 85% release after 48 hours studies was found to be within limits. First order drug release was observed with the fickian diffusion because the n value was less than 0.45. Similar values proved that F5 formulation was the best due to its release pattern.
Fanzhu Li
Zhejiang Chinese Medical University, China
Title: A novel doxorubicin loaded folicacid conjugated PAMAM modified with borneol, a nature dual-functional product to freducing PAMAM toxicity and boosting BBB penetration
Time : 12:40-13:00

Biography:
Fanzhu Li, male, doctor, professor, doctoral supervisor and postdoctoral cooperative tutor. In march 2002, Prof. Li was introduced to Zhejiang Chinese Medical University as a special talent. Now, he is the dean of college of pharmaceutical science. Prof. Li engaged in novel drug delivery system, targeting preparation, new dosage form and novel technique as well as the process of chinese medicine in vivo for a long time. At the same time, Prof. Li has established targeted drug delivery systems of brain, liver, kidney and other organs, studied new methods of administration of nasal mucosa, and first initiated a new model of the process of traditional Chinese medicine in vivo in china.
Abstract:
E​ffective targeting drug delivery system for glioma treatment is still greatly challenged by the existence of the blood-brain barrier (BBB) and the intracranial over spreading of anti-tumor drug. Herein, we presented a dual-functional glioma targeting delivery of doxorubicin based on the PAMAMG5 dendrimer, modified with folic acid (FA) to target tumor cell, alsoborneol(BO), a well known safe material derived from traditional Chinese medicine, to facilitate the BBB permeability and reduce the toxicity of naked PAMAM. The intracranial transportation and glioma targeting ability were evaluated on the BBB model and C6 glioma cells in vitro. Also, pharmacokinetics and biodistribution were studied on C6 glioma-bearing rats in vivo. It indeed reduced the cytotoxicity of PAMAM against both HBMEC and C6 cells by coupling BO on the surface, while efficiently boosted BBB permeability with the improvement of transportation ratio by 2 folds to the BO-unmodified conjugates. Furthermore, conjugated FA increased total uptake amount by C6 cells leading to strong inhibition with the 3-fold lower IC50 value than FA-unmodified DOX conjugate. In comparison with DOX solution, FA-BO-PAMAM/DOX exhibited significantly prolonged half-life time and increased area under the curve and improved DOX accumulation in brain tumor. The tumor growth inhibition, in vivo, was significantly increased up to 57.4%. The median survival time of xenograft rats after administering FA-BO-PAMAM/DOX (28 days) was significantly prolonged compared to free DOX (18 days, P < 0.05) or other controls. In conclusion, this strategy of novel targeting nanocarrier provides apromising method to increase the drug accumulation in the tumor site for therapy of glioma.
Zaheer Ahmad
Chinese Academy of Sciences, China
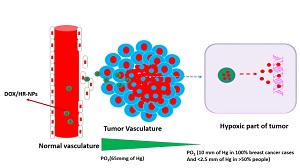
Title: Hypoxia-responsive methoxy poly (ethylene glycol)-block-poly (glutamic acid)-graft-6-(2-nitroimidazole) hexyl amine nanoparticles for doxorubicin drug delivery
Time : 13:00-13:20

Biography:
Zaheer Ahmad completed his PhD at Quaid-I-Azam University Islamabad, Pakistan in 2016. He performed most of his PhD research abroad at University of Alberta, Canada (under the supervision of Professor Mike Serpe) and Changchun Institute of Applied Chemistry (under the supervision of Professor Xuesi Chen). His research interest focuses on “Development of pH and hypoxia responsive poly amino acid based nanocarriers and its in vitro and in vivo investigation using breast cancer mice xenograft model. He published about seven peer-reviewed papers in international journals such as Journal of Controlled Release, RSC Advances and Macromolecular Biosciences.
Abstract:
Tumor micro environment-targeted nano drug delivery vehicles are gaining mounting attention in the field of biomedical sciences. The hypoxic response of the tumorous cells due to very low partial pressure of oxygen (sometimes less than 2.5 mm of Hg) in the tumor tissues makes hypoxia responsive drug delivery system as the more appealing in cancer chemotherapy. Based on these considerations, we synthesized hypoxia responsive polymeric materials methoxy poly(ethylene glycol)-block-poly(glutamic acid)-graft-6-(2-nitroimidazole) hexyl amine (mPEG-b-PLG-g-NID) by conjugation of the hydrophobic nitroimidazole derivative (NID) [6-(2-nitroimidazole) hexyl amine] with the pendant carboxylic group of poly(ethylene glycol)-block-poly(L-glutamic acid) (mPEG-b-PLG). The structure and degree of substitution were confirmed by proton NMR, FTIR and UV-Vis spectroscopy. The degree of substitution was found to enhance with the increase in nitroimidazole derivative to polymer ratio. The hypoxia response of the material was evaluated by UV-Vis spectroscopy and zeta potential measurements. Doxorubicin was hydrophobically encapsulated in the micellar core of the hypoxia responsive nanoparticles. The drug loaded micelles showed faster release in hypoxic condition as compared to normoxic conditions. Moreover, the developed polymeric system was found non-toxic to MCF-7 cell line thus suggesting its biocompatibility and suitability as drug delivery device.

Biography:
Mohammad Salhab is an Academic Clinical Fellow in Orthopaedics at the unit with a PhD studentship in pharmaceutics and currently involved in developing the NM. Mr Salhab has published many articles in surgical field and also bioethics and basic science.
Abstract:
Background: Acute pain control following elective primary total knee replacements (TKRs) and total hip replacements (THRs) is often poor and is associated with long term chronic pain syndrome. Moderate to severe pain is often reported in the first 48 hours following surgery requiring different pain modality management strategies such as patient controlled analgesia and multimodal drug analgesia. The Local Infiltration Anaesthetic (LIA) technique is currently an established technique to tackle perioperative pain relief; however, studies have reported conflicting evidence so far. In a recent review of 29 studies investigating the use of LIA in TKR, LIA emerged as a safe technique with improved pain control (Gibbs DMR 2012). We have developed the LIA technique to include an intra-articular catheter allowing an infusion of Novel Mixture (NM) to be infused continuously postoperatively.
Kuldeep H Ramteke
Modern College of Pharmacy, India
Title: Various potentials of isolated bael fruit gum in drotaverine hydrochloride tablets

Biography:
Kuldeep Ramteke has his expertise in the application of newly accepted optimization technique i.e. SeDeM and he is having the good practical hand regarding the isolation of the natural gums from the natural sources. The main emphasize is on the application of the isolated gum for the development of the new drug delivery system or new use of the natural polymer.
Abstract:
Back ground: Gum constitute a major group of naturally occurring polymer, their abundance and low cost make them the preferred choice in foods and pharmaceuticals. Bael (Aegle Marmellose linn) fruits are edible, and reported in ancient system of medicines for their various activities.
Aim: The present research work was conducted to evaluate the various excipients potential of bale fruit gum (BFG) for solid dosage form. Material and Methods: BFG was isolated from partially ripe fruits of Bael. Identification and Standardization of BFG was carried out as per the stated procedures in the pharmacopoeia. Studies were conducted to support the physicochemical properties of gum. Solid state characterization of BFG was done by SEM, FTIR, DSC and XRD techniques. Different formulations were prepared to check the excipients profile of BFG like disintegrant, diluents, binder, carrier for solid dispersion, phosphorylated derivative and combination with other natural superdisintegrant and lyophilized derivative. Drotaverine Hydrochloride (DTV) was used as model drug for this experiment. Different tablet formulations were prepared and evaluated for physicochemical properties like appearance, average weight, hardness, thickness, disintegration, assay and dissolution. Prepared formulations were optimized by using the SeDeM diagram expert system to check the direct compression suitability of the blend.
Result: It was found that BFG possess good compressibility, flowability, low moisture content, neutral pH, amorphous nature and good water solubility. This made it suitable to be used as the release modulator in various dosage forms. It also possesses the binder and disintegrant property. When BFG was lyophilized, its surface characteristics were changed. Increase in pore size and ultimately the porosity and decrease in particle size proved its use as the superdisintegrant in immediate release tablets. Comparison of the BFG and Lyophilized BFG (LBFG) physicochemical properties shows that LBFG possess superior disintegrant potential compare to BFG for immediate Release tablet.
Conclusion: From the research work it was concluded that LBFG in concentration range of 10-12% shows superdisintegrant activity.
Muyiwa Arisekola
Walter Sisulu University, South Africa
Title: Anti-inflammatory activity of the volatile oil of the pulp of Raphia australis

Biography:
Muyiwa Arisekola is an emerging student researcher. He finished his 5-year Bachelor of Technology degree in pure and applied chemistry with a second class (upper division). His vision is to parlay his passion for organic chemistry into a drug design and discovery career via a profound understanding of pathogenesis of diseases and structure-activity relationship of drugs. He is currently pursuing a degree of masters of science (by research) in organic(medicinal natural products) chemistry. He is preparing his first paper for publication.
Abstract:
Statement of the Problem: Corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs) have been used for decades in the treatment of inflammatory disorders. Corticosteroids have limitations some of which are depression, glucose intolerance, and susceptibility to infection due to depressed body immunity. NSAIDs also have their side effects due to the undesired depletion of COX-1. After the development of COX-2 specific inhibitors, researchers later discovered that COX-2 also is constitutively produced in the body. The limitations of these NSAIDs have necessitated a research into natural products which show potent anti-inflammatory activity while at the same time do not interfere with the actions of COX-1 and COX-2 which are expressed in some specialized body tissues. The purpose of this study is to look into the use of volatile oil of Raphia australis as an anti-inflammatory agent . Methodology & Theoretical Orientation: The volatile oil of Raphia australis was extracted in hexane using Clevenger Apparatus, after which the GC-MS analysis of the oil was carried out. The anti-inflammatory test was done using four groups (1, 2, 3, and 4) of rats comprising six rats per group. Group one were administered normal saline (0.09% v/v NaCl) while group1,2 and 3 were administered 40mg/kg(oil), 80mg/kg(oil) and 100mg/kg aspirin respectively. Inflammation was induced in all the groups using lipopolysaccharide thirty minutes after treatment by gavage. The major constituents obtained from the GC-MS analysis of the volatile oil are: n-hexadecanoic acid (34.0%), tetradecanoic acid (9.5%), terpinolone (9.1%), dodecanoic acid (9.6%), hotrienol (8.8%), alpha-terpine-1,4-diene (.8%), transbenzalacetone (8.2%), nonanoic acid (7.7%) and beta-ionone (6.0%), lauric acid (9.6%). Conclusion and Significance: The 80mg/kg dose of the oil showed a significant anti-inflammatory effect (P<0.01) when compared to 100mg/kg of aspirin (P<0.01). The analgesic activities of aspirin (P<0.05) and the 80mg/kg dose (P<0.05) of the oil were only significant in the inflammatory phase of the analgesic test.

Biography:
To be updated...
Abstract:
Motivation:-Cancer is a group of diseases involving abnormal cell growth with the potential to spread to other parts of the body. Chemotherapy is a type of cancer treatment that uses drugs to destroy cancer cells. Thus realizing the need to overcome complexities involved in treating complex diseases motivated the author to design casein coated iron oxide nanoparticles (CCIONPs) crosslinked with glutaraldehyde for achieving efficient MDT. Method: - In order to design casein nanoparticles (CCIONPs) the microemulsion method was adopted and for impregnation of iron oxide nanoparticles into the bulk of casein matrix an in situ co precipitation method was used. In order to characterize nanoparticles FTIR, XPS, TEM, SEM, VSM, Mossbauer, zeta potential, in-vitro cytotoxicity test were studied. In order to investigate the drug release behaviour of drug loaded CCIONPs the magnetic fields of strength 1000G, 1500G, 2000G, 3000G, 5000G and 5500G were applied. The nanoparticles were loaded with cytarabine and its controlled release was investigated drug loading, chemical architecture of the nanocarriers, and nature of release media. In vivo analysis was perfomed on mice model and different cell lines. Results: - FTIR analysis confirmed homogenous deposition of iron oxide and subsequent formation of CCIONPs. The drug loading efficiency of CCIONPs, drug content and in vitro drug release profiles may be measured by ultraviolet spectrophotometer at λmax 254. It was found to have better payload, in vitro release profile characteristic and better targeting to RES organs. Conclusion: - Glutaraldehyde crosslinked casein coated iron oxide nanoparticles CCIONPs form a swelling controlled drug release system, which effectively delivers cytarabine in the presence and absence of magnetic field via diffusion controlled pathway. Thus, the prepared nanoparticles showed potential to provide a possible option for magnetically targeted delivery of anticancer drugs.
Tesfaye Zerihun
Addis Ababa University, Ethiopia
Title: Methalonic extract of the exudate of Aloe otallensis and its effect on Leishmania Eathiopica Parasite and its phytochemical screening

Biography:
I am working as a Senior Clinical Pharmacist at Addis Ababa University, college of Health Science, Black lion specialized Teaching Hospital In this teaching Hospital I am serving as Drug Supply Management coordinator, The Head of special Pharmacy of the hospital, the secretary of DTC (Drug therapeutic Committee) and other committee works. In addition to these Mentoring under graduate pharmacy students who are coming to the hospital for clinical attachment both at the ward and dispensary area .I am also participating in some of Clinical research which is under go in the Hospital beside the routine work.
Abstract:
Background & objectives: Several plant products have been tested and found to possess antileishmanial activity. The present study was undertaken to evaluate antileishmanial activity of methanolic extract of Aloe otallensis on the promastigot stage of Leishmania aethiopica comparing to standard drugs and also tried to screen its phytochemical constitute.
Methods: Phytochemical screening was done using the method mentioned by Evan and Trease on methanolic extract exudates of Aloe otallensis leaf. The extract was also evaluated for in vitro antileishmanial activity against Leishmania Aethiopica which is found from the black lion hospital parasitology unit. The result was compared to standard drug of Sodium stibogluconate, milfostin and paramomycin.
Result: The extract has a good antileishmaniacidal activity with an IC50 of 0.041 μg/ml on L. aethiopica (LDC/134). The experimental data shows that relatively it has better activity than paramomycin and milfostin but less activity than sodium stibogluconate. The data analyses was done by pad graph prison version 5 software after it was read by ELISA reader at the wave length of 650 nm. The phytochemical screening of the exudates of aloe otallensis showed the presence of phenol, alkaloid and saponin.
Conclusion: The methanol extract of exudate of Aloe otallensis has a good anti leishmanisis activity and this may be attributed to phenol, alkaloid and saponin presnt in the plant. But it needs further analysis for the conformation of which constituent present in much concentration and to know which one have highest role.
Rashid Mahmood
Surge Laboratories Private Limited, Pakistan
Title: Hot melt extrusion an emerging drug delivery technology

Biography:
Rashid Mahmood has Master Degree in Analytical Chemistry and MS in Total Quality Management. He has 13 years of experience of Pharmaceutical Quality Ope rations and has attended many international conferences as a keynote speaker and presented various talks in USA & China on Cleaning Validation, cGMP Guidelines, Quality Risk Management etc. Currently he is working as a Senior Executive Manager Quality Operation for Surge Labs.(Manufacturer of Microencapsulated APIs, Liquid & Dry Pow der Parentrals) which is the best export oriented company in Pakistan.
Abstract:
Over the last three decades hot-melt extrusion (HME) has emerged as an influential processing technology in developing molecular dispersions of active pharmaceutical ingredients (APIs) into polymers matrices and has already been demonstrated to provide time controlled, modified, extended and targeted drug delivery resulting in improved bioavailability. HME has now provided opportunity for use of materials in order to mask the bitter taste of active substances. Since industrial application of the extrusion process back in the 1930’s HME has received consider able attention from both the pharmaceutical industry and academia in a range of applications for pharmaceutical dosage forms, such as tablets, capsules, films and implants for drug delivery through oral, transdermal and transmucosal routes. This makes HME an excellent alternative to other conventionally available techniques such as roll spinning and spray drying. In addition to being a proven manufacturing process, HME meets the goal of the US Food and Drug Administration's (FDA) process analytical technology (PAT) scheme for designing, analyzing as well as controlling the manufacturing process through quality control measurements during active extrusion process. The use of hot-melt extrusion (HME) within the pharmaceutical industry is steadily increasing, due to its proven ability to efficiently manufacture novel products. HME involves the application of heat, pressure and agitation through an extrusion channel to mix materials together and subsequently forcing them out through a die. Twin-screw extruders are most popular in solid dosage form development as it imparts both dispersive and distributive mixing. It blends materials while also imparting high shear to break-up particles and disperse them. HME extrusion has been shown to molecularly disperse poorly soluble drugs in a polymer carrier, increasing dissolution rates and bioavailability.
Abdullah Shamim
Khulna University, Bangladesh
Title: Genetic polymorphisms of CYP3A4*1B of cervical cancer patients in Bangladeshi population, Bangladesh

Biography:
To be updated...
Abstract:
Background: Cervical cancer incidence rate in Bangladesh is 15.9 and age-standardized incidence rate is 19.2 (rates per 1,00,000 women per year). Polymorphisms of different genes have been affirmed to be associated with cervical cancer. Our prime purpose is to observe whether CYP3A4*1B polymorphisms are related with increasing cervical cancer risk in Bangladeshi population. The compilation of precise clinical information allows the clarity of different clinical phenotypes, which play a vital role in genetic studies of cervical cancer.
Methods: The study was a case-control study carried out between patients and volunteers matched by age, sex, height, weight and smoking status. Daly’s chemical method was used to isolate genomic DNA from venous blood. The demographic variables of cases and controls were put side by side using chi-square tests and Student’s tests.
Results: Odds ratio and 95 % confidence interval were assigned to estimate the risk of cervical cancer. CYP3A4*1B polymorphisms and cervical cancer risk do not show any considerable relationship. The polymorphic frequencies of CYP3A4*1B allele (normal homozygote, heterozygote and mutant homozygote) in cervical cancer were 60%, 40% and 0% respectively; frequencies in control were 66.67%, 33.33% and 0% respectively (p<0.05). Finally, we conclude that CYP3A4*1B polymorphisms is not associated in susceptibility to developing cervical cancer, at least in Bangladeshi population.
Conclusions: In brief, the consequences of our study demonstrated that CYP3A4*1B polymorphism is not allied in susceptibility to develop cervical cancer, at least in Bangladeshi women.
Tesfaye Zerihun
Addis Ababa University, Ethiopia
Title: Phytochemical screening of the exudate of Aloe otallensis and its effect on Leishmania donovani Parasite

Biography:
To be updated...
Abstract:
Objective: To evaluate antileishmanial activity of methanolic extract of Aloe otallensis (A. otallensis) on the promastigote stage of Leishmania donovani (L. donovani) as compared to standard drugs and to screen its phytochemical constituents.
Methods: Phytochemical screening was done by using the method mentioned by Evans and Trease on methanolic extract of the exudates of Aloe otallensis leaves. The extract was also evaluated for in vitro antileishmanial activity against L. donavani which is found from the Parasitology Unit of Black Lion Hospital. The result was compared to standard drugs of sodium stibogluconate, milfostin and paramomycin.
Results: The extract has a good antileishmanial activity with an IC50 of 0.123 0 μg/mL on L. donovani (AM 563). The experimental data showed that relatively it had better activity than paramomycin and milfostin but less activity than sodium stibogluconate. The data analyses were done by GraphPad Prism version 5 software after it was read by ELISA reader at the wave length of 650 nm. The phytochemical screening of the exudates of A. otallensis showed the presence of phenol, alkaloid and saponin.
Conclusions: The methanol extract of the exudates of A.otallensis has a good anti- leishmaniasis activity and this may be attributed to phenol, alkaloid and saponin present in the plant. But it needs further analysis for the conformation of which constituent presents in high concentration to know which one has the strongest effect.
Akbar Esmaeili
Islamic Azad University, Iran
Title: Vancomycin loaded superparamagnetic MnFe2O4 nanoparticles coated with PEGylated chitosan to enhance antibacterial activity

Biography:
To be updated...
Abstract:
Background & Aims: Increasing prevalence of antibiotic-resistant and failed-treatment make more investigations to deal with these problems. Hence, new therapeutic approaches for effective treatment are necessary. Ferrite superparamagnetic nanoparticles have potentially antibacterial activity.
Methods: In this study, we prepared MnFe2O4 superparamagnetic nanoparticles as core by precipitation method and used chitosan cross linked by glutaraldehyde as shell, then modified with PEG to increase stability of particles against RES.
Results: Chitosan coating not only improves the properties of ferrite nanoparticles but also has antibacterial activity. FT-IR confirmed this surface modification; XRD and SEM were developed to demonstrate particle size and characteristics of crystal structure of these nanoparticles. Final particle size was reported approximately 25 nm. Magnetic properties of nanoparticles were evaluated by VSM. Actual drug loading and releasing were examined by (UV-Vis) spectroscopy method.
Conclusions: We employed liquid broth dilution method to assess antibacterial activity of nanoparticles against microorganisms. Significant antibacterial effect against gram negative bacteria was developed.
