Day 2 :
Keynote Forum
Hyungil Jung
Yonsei University, South Korea
Keynote: Microneedles: The future of painless drug delivery systems
Time : 10:00-10:35

Biography:
Hyungil Jung completed his PhD from Cornell University and his Post-doctoral studies from California Institute of Technology (Caltech). Since then he has received various awards such as “Outstanding Contributions”, “Best Contribution Award”, “Excellence in Research Award”, “The 31st Industry-academic Cooperation Award”, “Best technology Award”, “Best Teaching Award” and many more in the field of Biotechnology, because of his outstanding research ability in the field. He has also recently registered his company, Juvic Inc., to further expand his research and to introduce novel microneedle based pharmaceutical and cosmeceutical products in the market.
Abstract:
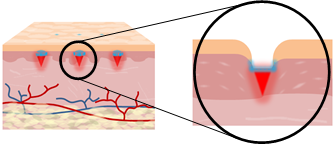
Microneedles are micro dimensional needles capable of delivering biological therapeutics as well as cosmetics into the skin without causing pain, in a minimally invasive manner. In addition, microneedles are referred as the future of drug delivery systems due to their advantages compared to currently utilized drug delivery routes including topical application and hypodermic injection. There are various types of microneedles including solid type, hollow type and dissolving type. Solid microneedles are used to create pores onto the skin by which the therapeutics can be delivered with a higher efficiency. Hollow microneedles are micro scale hypodermic needles that are less painful than normally used needles. Dissolving microneedles which have been receiving big attention in recent years are referred to a type of microneedle that encapsulates drugs within its polymer and delivers it into skin upon insertion through dissolving process. Each of these microneedle types, based on the application purposes can be applied in different branches of drug or cosmetic compounds delivery. Through microneedles, achievement of a highly efficient delivery has become possible and we are expecting microneedles to replace the widely used hypodermic needles in the near future. We have so far developed various dissolving microneedle fabrication methods by which activity of encapsulated therapeutics within microneedles can be maintained the most. Centrifugal lithography (CL) is one of the recently developed fabrication methods that can be used for the fabrication of microstructures by a single centrifugation, and engineering the self-shaping properties of hyaluronic acid (HA). We have also developed microneedle implantation systems by which dissolving microneedles can be fully inserted into the skin in a minimally invasive manner.
Keynote Forum
Kostas D Demadis
University of Crete, Greece
Keynote: Smart, programmable and responsive injectable hydrogels for controlled release of cargo osteoporosis drugs
Time : 10:35-11:10

Biography:
Kostas D Demadis is a Full Professor in the Department of Chemistry, University of Crete, Greece and Head of the Crystal Engineering, Growth & Design Laboratory. His research group is interested in a number of research areas such as coordination polymers with emphasis on metal phosphonate MOFs, functional polymers, silicon chemistry (modeling of biosilicification mechanisms), water treatment issues (mineral scale inhibition, corrosion control, metal ion absorption), controlled release of active ingredients (in particular bisphosphonate drugs), “green” chemistry, and hybrid polymeric materials for cultural heritage protection. He has published ~150 papers in peer reviewed journals, about a dozen chapters in books, four books, and is the inventor of two patents.
Abstract:
Gel systems have found extensive applications in the medicinal/pharmaceutical field because of their ease of preparation, ability for modifications and responsiveness to external chemical or physical stimuli. Gels usually act as hosts for active pharmaceutical agents for a variety of pathological conditions. Among the known bone diseases (osteoporosis, osteoarthritis, multiple myeloma, Paget’s disease and several others), the most challenging is osteoporosis, which burdens millions of people compromising patients’ quality of life. The recommended pharmaceutical treatment is the use of bis-phosphonates (BPs, a.k.a. “-dronates”). Their success in mitigating osteoporosis, notwithstanding these “-dronate” drugs present a number of challenges including fast excretion, and numerous side-effects, such as osteonecrosis of the jaw, hypocalcemia, esophageal cancer, ocular inflammation, atrial fibrillation, etc. Nevertheless, the main drawback of BPs is their limited oral bioavailability. It is, therefore, imperative to design and fabricate “smart” systems that allow controlled delivery of the active BP agent, which will depend on the patient’s needs and idiosyncrasies. In this presentation we discuss easy-to-prepare drug delivery systems, based on smart, silica gels. These have been synthesized, characterized, and studied as hosts in the controlled release of several bisphosphonate drugs. They exhibit variable release rates and final % release, depending on the nature of bisphosphonate (side-chain length, hydro-philicity/-phobicity, water-solubility), cations present, pH and temperature. These gels are robust, injectable, re-loadable and re-usable.
- Nanoparticulate Drug Delivery Systems | Biomedicine and Pharmacotherapy Pharmaceutical Nanotechnology | Nanomedicine and Nanotechnology
Location: Zieten

Chair
Hyungil Jung
Yonsei University, South Korea

Co-Chair
Kostas D Demadis
University of Crete, Greece
Session Introduction
Margitta Dathe
Leibniz-Forschungsinstitut für Molekulare Pharmakologie, Germany
Title: Peptide-modified micelles and liposomes: Carriers for xenon hyper-CEST MRI of blood brain barrier endothelial cells
Time : 11:30-11:55

Biography:
Margitta Dathe studied Physics at the Humboldt University of Berlin and completed her PhD in 1978 from the Academy of Sciences of the GDR. Since 1999, she has been working as Head of the Peptide-Lipid Interaction Research Group of the Leibniz Research Institute of Molecular Pharmacology. Her research interest is focused on targeting, cellular uptake promoting peptides and lipid-based carrier systems as well as on antimicrobial peptides. She has published more than 100 papers in reputed journals
Abstract:
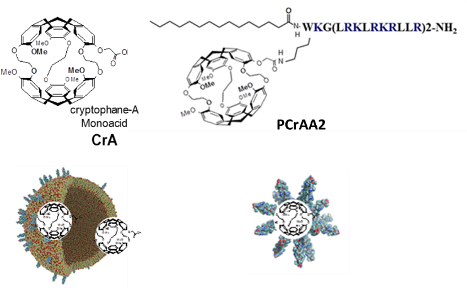
Selective imaging of pathological areas and targeted drug delivery are crucial for efficient diagnostics and therapy. Drug delivery to the brain is a particular challenge. We generated highly cationic lipopeptides that form micelles and bind to liposomes. Cargos, covalently bound or incorporated into such carriers are selectively transported into blood brain barrier endothelial cells. Basis for the selective uptake of the different systems is the activation of clathrin-mediated endocytosis, a process which is not addressed in other vessel endothelial cells. Here we present the development of peptide-modified micellar and liposomal carriers for the selective transport of cryptophane-A (CrA) into human brain capillary endothelial cells. Chemical exchange saturation transfer with hyperpolarized xenon nuclei (Hyper-CEST) allows highly sensitive detection of supramolecular cages such as CrA in non-invasive Magnetic Resonance Imaging (MRI). Incorporation into liposomes distinctly reduced the toxicity of the hydrophobic CrA and a one nanomolar concentration generated sufficient contrast to distinguish between brain capillary and aortic endothelial cells. Covalent attachment did not influence the micelle characteristics and provided additional advantages as it results in high local cage concentration and allows more reliable quantification of the signal molecule. The peptide-modified carriers combine a high selectivity for human brain capillary endothelial cells with the great sensitivity of Xe Hyper-CEST MRI and might be a promising MRI tool.
Scheme of peptide-tagged CrA-loaded liposomes and PCrAA2 micelles for Xenon Hyper-CEST MRI
Ruba Bnyan
Liverpool John Moores University, UK
Title: Transfersomes as novel carriers for sustained buccal delivery of local anaesthetic
Time : 11:55-12:20
Biography:
Ruba Bnyan is a PhD student in the Formulation and Drug Delivery Research Group, School of Pharmacy and Biomolecular Sciences, Liverpool John Moores University. Her research interests are generally in designing drug delivery system, nanotechnology and pharmaceutical technology, focus being on preparing non-invasive delivery systems for several routes of administrations.
Abstract:
The aim of this work was to design and develop a delivery system to treat dental and buccal pain. Transfersomes show many advantages as delivery vesicles such as their ability to deform and pass through small pores between cells, and to encapsulate drugs with a wide range of solubilities and molecular weights. The rationale of preparing sustained release transfersomes of local anaesthetic (LA) was mainly to reduce the frequency of administration and enhance the safety profile of LA by producing a localized effect. Transfersome preparation parameters were optimized using a Taguchi design of experiment (DOE) in terms of phospholipid to edge activator (EA) ratio, type of EA and type of lipid. The delivery systems were characterised for vesicles size, polydispersity index (PDI), charge, and entrapment efficiency (EE). They were generally less than 200 nm in size with a low PDI. The %EE varied as the formulation parameters changed, but was generally between 44-50%. Analyzing the data by Taguchi DOE showed that the effects of factors on both size and %EE were in the following rank: EA type˃lipid: EA ratio˃lipid type. Samples that showed higher encapsulation with smaller vesicles size were chosen for further studies. In vitro release studies were performed using a dialysis bag (3-5 kDa) as a donor compartment, which was sealed and placed in a receptor compartment containing PBS. The system was stirred at 250 rpm and incubated at 37oC. Initial in vitro release results showed a sustained release over 72 hours.
Janina-Miriam Noy
The University of New South Wales, Australia
Title: Direct polymerization of the novel arsenic drug PENAO to obtain polymeric nanoparticles for the treatment of sarcoma
Time : 12:20-12:45

Biography:
Janina-Miriam Noy has completed her Bachelor’s and Master’s Degree at the Heinrich-Heine University in Düsseldorf (Germany) before she relocated to Sydney (Australia) in 2013. She worked as a Research Assistant for two years at the Centre of Advanced Macromolecular Design (CAMD) at the University of New South Wales, focusing on the development of new stimulus-responsive materials for ‘smart’ drug delivery systems. Since August 2015, she is undertaking her PhD with the focus on the delivery of novel organicarsenical anti-cancer agents within polymeric nanoparticle formulations. She particularly investigates her arsenic containing drug-delivery systems towards sarcoma cells.
Abstract:
Recent investigations have shown the anti-cancer efficiency of PENAO, a second generation hydrophilic organoarsenical, towards a range of cancer cell lines and in a phase I/IIa dose escalation study in patients with solid tumours. However, the efficacy of PENAO – like most metal-based drugs – is limited by several factors such as high systemic toxicity, development of drug resistance, and rapid deactivation by complexation with proteins or oxidation reactions. Conjugation of drugs to a nanocarrier is an alternative strategy that overcomes many of these limitations. Polymeric nanoparticles for the delivery of chemotherapeutics has been widely proposed to prolong circulation time in the blood stream, to increase the specific retention in solid tumour tissue (enhanced permeability and retention (ERP) effect), and to avoid the recognition by the mononuclear phagocyte system. Herein, the direct synthesis of polymeric micelles, based on the novel arsenic drug PENAO is presented. PENAOs arsenous acid residue remains active when incorporated into a polymer matrix and conjugates to small mono and closely spaced dthiols, showing no significant difference in efficiency between PENAO containing polymers, PENAO containing nanoparticles and PENAO itself. Furthermore, the more stable micelle structures induce apoptosis in sarcoma cells and enhanced cytotoxicity and cellular uptake compared to the free drug. As a result, PENAO containing nanoparticles show great potential for further investigations into the biomedical arena and increasing the concentration of PENAO within the polymeric nanoparticle could improve antitumor efficiency, which leads to an auspicious outcome towards sarcoma cells.
Ilayda Acaroglu Degitz
Yeditepe University, Turkey
Title: Paclitaxel release from magnetite crosslinked polypropylene fumarate nanoparticles
Time : 14:45-15:10

Biography:
Ilayda Acaroglu Degitz has completed her MSc from Yildiz Technical University in 2013 and has been pursuing her PhD Degree at Yeditepe University in the Chemical Engineering Department since 2014. She has been working on nanoparticles, magnetic nanoparticles, drug delivery systems, drug and polymer conjugates. She has published two papers on nanoparticles. She is working as a Teaching and Research Assistant at Yeditepe University
Abstract:
In the treatment of cancerous cells, the most common treatment, chemotherapy, involves high toxicity and non-specific targeting in the body which leads to both cancerous and non-cancerous cells, inside and outside the tumor becoming damaged or destroyed due to the agents spreading throughout the body which further limit the productivity. Therefore, attempts are more focused on targeting cancer therapeutics, thus reaching the objective of developing new carrier systems with both existing and new drugs. Magnetic drug delivery using drug carriers such as biodegradable polymers, is a very efficient method to target the drug to a localized disease site in the body. The aim of this study was to develop a new magnetic drug delivery system. For this purpose, magnetic nanoparticles were incorporated in biocompatible polymers namely polypropylene fumarate (PPF) which was crosslinked with an N-vinyl pyrrolidone (NVP) using photo initiated miniemulsion polymerization method. FTIR spectroscopy was used to confirm the crosslinking of PPF with NVP, and swelling tests were used to determine the percentage of crosslinking. Then the morphology and size of crosslinked polymer particles with and without magnetite were determined by scanning electron microscopy (SEM). Crosslinked polymer nanoparticles with embedded magnetic nanoparticles were loaded with paclitaxel (anticancer drug). The amount of drug released from crosslinked polymer nanoparticles with and without magnetite was determined using high-performance liquid chromatography (HPLC) and these carriers were found to release all the encapsulated drugs in less than one month, before the polymer nanoparticles were degraded. Thus, it suggests the drug release to be diffusion controlled.
Hassan Bardania
Yasuj University of Medical Sciences, Iran
Title: Cell targeting small peptides as smart ligands for targeted drug delivery
Time : 15:10:15:35
Biography:
Hassan Bardania has completed his PhD from Tarbiat Modares University, Iran. He has published more than 10 papers in reputed journals.
Abstract:
Targeting ligands are used in drug delivery to decrease the side effects and increase the bio-availability of drugs. Small peptides with high affinity for special receptors have attracted more attention as a new class of ligands to deliver specifically therapeutic and diagnostic agents. They are typically identified by using phage display and chemical synthetic peptide library methods. In comparison with other conventional ligands such as antibodies, these ligands have several advantages including easy synthesis, smaller physical sizes, lower immunogenicity and cytotoxicity and their simple and better conjugation to nano-carriers and therapeutic or diagnostic agents. In our research, we have used small peptides as a targeting ligand to deliver therapeutic agents to activated platelets and cancerous cells. In our previous study, we used nanoliposoms modified with a motif of RGD peptide to deliver eptifibatide (as an anti-platelet drug) to activated platelets. The in vitro and in vivo results showed that encapsulation of eptifibatide into RGD-modified nanoliposomes significantly improved platelet aggregation inhibitory activity of drugs compared to free drugs. In our next study, we will be using small peptides as targeting ligands for drug targeting to cancerous cells.