Day 2 :
Keynote Forum
Arwyn T Jones
Cardiff University. UK
Keynote: Overcoming Cellular Barriers for Drug Delivery: Opening Endocytic Gates and Pathways for Intracellular Targetting
Time : 09:30-10:10

Biography:
Arwyn gained his PhD in protein biochemistry and crystallography at Birkbeck College, University of London. Then he undertook postdoctoral positions investigating endocytosis at the University of Liverpool and Harvard University, Boston USA. In 2000 he was awarded a European Molecular Biology Organization fellowship to work at the European Molecular Biology Laboratory (EMBL), Heidelberg Germany, and continued at the EMBL when he was awarded an Alexander von Humboldt Foundation Scholarship. He was appointed as Lecturer at the Cardiff School of Pharmacy and Pharmaceutical Sciences at Cardiff University in 2002 where he is now a Professor in Membrane Traffic and Drug Delivery.
Abstract:
Targeting a disease process inside a cell with biopharmaceuticals still represents a major challenge, not least in overcoming biological barriers such as those posed by the plasma membrane. Investment in this approach is justified when one considers the number individual intracellular targets now available to us as we continue to understand disease processes at the gene and protein level. This is true for many high-burden diseases including cancer, infectious diseases and inherited genetic defects such as cystic fibrosis. Our research is focused on studying endocytosis and specifically on designing methods to analyse individual endocytic pathways to characterise how drug delivery vectors and associated therapeutics gain access to cells. As vectors we have paid particular attention to natural ligands, cell penetrating peptides and antibodies, focusing on their capacity to not only interact with, and enter cells, but then on monitoring their intracellular traffic to reach a final destination. In this lecture I will describe work we have performed focusing on design and characterization of methods to study endocytosis of drug delivery vectors and on recent studies showing how internalisation of plasma membrane receptors can be significantly enhanced, and their normal endocytic routes modified to reach a desired intracellular location. Our involvement in a €30M FP7 Innovative Medicine Initiative (IMI-EFPIA) consortium (COMPACT www.compact-research.org/) will also be discussed. This represents a public-private collaboration between 14 European academic institutes and pharmaceutical companies aiming to improve the cellular delivery of biopharmaceuticals across major biological barriers of the intestine, lung, blood brain barrier and skin.
Keynote Forum
Raid Alany
Kingston University, UK
Keynote: Age-related sight loss: novel drug delivery strategies to the anterior and posterior segments of the eye
Time : 10:10-10:50

Biography:
Professor Raid Alany has over 25 years of international experience in pharmacy education, pharmaceutics and drug delivery research. His academic journey spans three continents, namely, Asia, Oceania and Europe. He received his PhD in drug delivery from the University of Otago, Dunedin New Zealand in 2001; was appointed as a Lecturer at the School of Pharmacy, The University of Auckland, Auckland, New Zealand. He joined Kingston University London as Professor (Chair) of Pharmaceutics in January 2011 and was appointed as Research Director for the School of Pharmacy and Chemistry in December 2013. Raid is an author on over 200 scientific research publications (papers and abstracts), a book and seven book chapters. Professor Alany acts as Editor-in-Chief for Pharmaceutical Development and Technology, Section Editor for Clinical and Experimental Ophthalmology the official journal of the Royal Australian and New Zealand College of Ophthalmologists; Chief Patron, Drug Development and Therapeutics, a publication of Organization of Pharmaceutical Unity with BioAllied Sciences (OPUBS). He serves on the Editorial Board of the following journals: Current Medical Research and Opinion, BioMed Research International, Journal of Drug Delivery Science and Technology, Current Drug Delivery, Pharmaceutics MDPI, and Drug Delivery Letters. He is the Immediate Past President of the New Zealand Chapter of the Controlled Release Society (NZCRS), Young Scientist Committee of the Controlled Release Society. Raid won several awards such as Microscopy New Zealand Young Scientists Award in 1999 The University of Auckland's Vice Chancellor's Early Career Research Excellence Award in 2003, the Controlled Release Society Veterinary Programme co-chair/ chair Distinguished Service Awards in 2008/2009 and the Spark Ideas Challenge, Uniservices Prize and Chiasma Prize in 2011. He consults for human and veterinary pharmaceutical companies in New Zealand and Singapore and is an inventor on several international patents.
Abstract:
Aging is associated with drastic optical and biochemical changes in the eye often leading to a decline in visual acuity where vision worsens. Such eye disorders impose a financial burden on the health sector worldwide. Recent estimates of the global cost of sight loss -up to the year 2010- suggest an annual figure of over US$3 trillion (£2.4 trillion). The main disorders leading to sight loss are cataract, glaucoma, age-related macular degeneration (AMD) and diabetic retinopathy. Pharmaceutical formulation and drug delivery research has introduced promising eye treatments into the market; nevertheless, there remain unmet clinical needs and limitations associated with performance of conventional eye drops and ointments. Compromised adherence and/or persistence with conventional eye drops that are applied topically to the surface of the eye is primarily related to the need to be applied once, twice (or even up to four times) daily, often as a combination of multiple drugs, to achieve their intended purpose. The intravitreal injection of anti-vascular endothelial growth factor (VEGF) for AMD treatment requires clinical intervention every 4-8 weeks. Therefore, achieving therapeutics drug concentrations at the target site and maintaining such concentration over extended time intervals with minimal undesirable effects, offer renewed opportunities for product research and development, especially when using already approved drugs with well-established safety and efficacy profiles. This talk will review and provide insights withdrawn from our own research on ophthalmic drug delivery systems that are aimed at age-related eye disorders including phase-transition microemulsions, in-situ gels, polymeric and inorganic nanoparticles, personalised ocular inserts and modified contact lenses.
Keynote Forum
Sharareh Salar-Behzadi
Research Center Pharmaceutical Engineering GmbH, Austria
Keynote: Lipid-based pharmaceutical formulations for patient-centric product development
Time : 11:10-11:50

Biography:
Sharareh Salar-Behzadi held her diploma in Pharmacy and PhD in Pharmaceutical Technology from University of Vienna. Her experience covers a broad range, including formulation and process development for production of solid dosage forms. She worked on several pharmaceutical manufacturing methods, among them solvent free hot-melt fluid-bed technology, wet fluid-bed granulation, roller compaction and methods for development of nano lipid carriers. She works at Research Center Pharmaceutical Engineering (RCPE) GmbH since 2012 as Project Lead for scientific execution of projects for formulation engineering and development of particulate dosage forms. An important research focus is development of personalized-medicine with advanced stability, based on lipid-based excipients
Abstract:
Statement of the Problem: Lipids and lipid-based excipients are increasingly applied for development of patient-centric products. Their application in the pharmaceutical formulations covers a wide range, from taste-masking of oral dosage forms with modified; both immediate- and extended release profile to development of advanced nanoparticles for pulmonary or parenteral route of drug administration. Despite of this diversity in application, the drug release instability and the lack of mechanistic understanding of it still prevent the larger-scale application of lipidic excipients. This abstract provides a comprehensive overview on the complex solid state behavior of lipids and describes methods for monitoring this behavior for obtaining reliable and reproducible dosage forms.
Methodology & Theoretical Orientation: Solid state behavior of lipids was studied as the response to the composition of formulation and to the critical parameters of the applied product manufacturing process, using X-ray diffraction, PLM and DSC. The applied processes were hot-melt coating for taste-masking and high pressure homogenization for preparation of nanosuspensions. Quality by Design (QbD) tools were used for monitoring the manufacturing process.
Findings: The instability of lipidic formulations can be addressed to both changes in molecular and supra-molecular levels. Changes in molecular level mainly contains polymorphic transformation and alteration in crystallite thickness, which can be monitored by careful selection of formulation composition and process parameters. Certain surfactants can be used as modifier, influencing the kinetic character of polymorphic transition of lipids. Process temperature can be monitored to control both crystallite growth kinetics and polymorphic transition. Understanding the microphase separation of formulations containing emulsifier is necessary and will help to improve the selection of pharmaceutical formulations.
Keynote Forum
Bruno Sarmento
Universidade do Porto, Portugal
Keynote: Nanoparticles-in-vaginal films for combined delivery of anti-HIV microbicide drugs
Time : 12:30-12:50

Biography:
Bruno Sarmento completed his PhD in Pharmaceutical Technology and Degree in Pharmaceutical Sciences at University of Porto, Portugal. He is an Affiliated Researcher at Institute of Investigation and Innovation in Health (i3S) and Institute of Biomedical Engineering (INEB), University of Porto, Portugal. He is an Assistant Professor of Pharmaceutical and Biopharmaceutical Technology at IUCS, Portugal. His current research is focused on “The development of functionalized nanomedicines and their application in the pharmaceutical and biomedical fields; in particular, nano-formulations of biopharmaceutical drugs with interest in diabetes, cancer and infectious diseases”. He has also specialization in “Mucosal tissue engineering models to validate functionalized nanomedicines and to perform in vitro/in vivo correlation”. He has published more than 160 papers in international peer reviewed (ISI) journals, 34 book chapters and more than 180 proceedings. He edited four books, participated in more than 50 invited/selected talks in national and international meetings and was awarded several distinctions. He is a member of Editorial Advisory Board of 10 international journals and has acted as referee for top-ranked journals in his area of expertise and for international funding agencies.
Abstract:
There is an urgent need to reinforce battle against HIV/AIDS, namely by investing more in preventing new infections. Scientific and medical evidence produced over recent years supports that both oral and topical pre-exposure prophylaxis (PrEP) are promising approaches that can reduce sexual transmission of the virus. Still, anti-retroviral with different physicochemical properties may be challenging to combine in one single microbicide product We propose a new system comprising the incorporation of nanoparticles (NPs) into films for the combined vaginal delivery of hydrophobic/hydrophilic molecules. EFV-loaded poly (lactic-co-glycolic acid) NPs were incorporated alongside free TFV into fast disintegrating films during film manufacturing. The delivery system was characterized for physicochemical properties, as well as for genital distribution, local and systemic 24 h pharmacokinetics, and safety upon intra vaginal administration to mice. EFV NPs with diameter of 145 nm were incorporated into a film with TFV. The film was soft and flexible. Disintegration time in simulated vaginal fluid was 9 min, resulting in dispersions with osmolality values near physiologic and pH of 4.24±0.02. The film presented low toxicity to CaSki, HEC-1-A and HeLa cells. NPs were evenly distributed and retained upon vaginal administration to mice. Mild epithelial penetration of NPs was observed. Drug concentrations in vaginal lavages and tissues peaked rapidly after film administration but slowly decreased up to 24 h. Still, drug concentrations were maintained at potentially protective levels. Films were found safe after daily 14 days administration as assessed by histological observation and analysis of IL-1b, IL-6, KC and TNFα levels.
Keynote Forum
Dagmar Fischer
Friedrich-Schiller University, Germany
Keynote: Bacterial nanocellulose as controlled drug delivery system in skin applications
Time : 11:50-12:30

Biography:
Dagmar Fischer is a Pharmacist. She has more than 20 years of experience in the field of “Nanocarriers based on synthetic and natural polymers, their formulation and biopharmaceutical characterization”. Furthermore, she has long-standing successful co-operations with many partners in and outside of Europe, in the field of Nano-safety. After completing her PhD in Habilitation at University of Marburg, she joined a biotech company for several years as Head of Preclinical Research and Development. In 2008, she was appointed as a Professor of Pharmaceutical Technology at University Jena.
Abstract:
The natural hydro-polymer bacterial nano cellulose (BNC) is an innovative biomaterial, produced during fermentation by strains of gram-negative bacteria Komagataeibacter xylinus and consisting of about 1% cellulose and 99% water. Although the chemical formula is identical to plant cellulose, the material favors totally different but outstanding material characteristics due to the three-dimensional network of nano-sized fibers. The interest in BNC as drug delivery system dramatically increased during the last years, as the nano-sized 3D-network of BNC is expected to hold a large amount of drug molecules due to its large surface area. However, the highly hydrophilic character limited a broad application especially for the delivery of lipophilic drugs as well as long-term applications. We developed different loading techniques to accomplish a controlled release of drugs from several hours to weeks using BNC produced under lab-scale as well as under high throughput conditions. Native BNC, hybrid systems with different types of the thermo-responsive block-copolymers poloxamers as well as lipid-modified BNC were established. Depending on the type of modification, not only the drug release profile, but also superior material properties such as high compression stability and water binding could be achieved. Using the antiseptic octenidine as model drug, the antimicrobial activity against Staphylococcus aureus and Pseudomonas aeruginosa was not changed by the use of the modified BNC. Excellent biocompatibility of the loaded BNC could be demonstrated after local administration in a shell-less hen’s egg model. In conclusion, controllable short- and long-term delivery systems consisting of poloxamer and lipid modified BNC could be developed as ready-to-use systems e.g. for dermal wound treatment, cosmetics or the use as implants.
Keynote Forum
Satyanarayana Somavarapu
UCL School of Pharmacy, UK
Keynote: Therapeutic potential against metastatic melanoma of a copper-based aquaporin inhibitor nanoformulated
Time : TBA

Biography:
Somavarapu move to England came with the award of a Commonwealth Fellowship from the Association of Commonwealth Universities and he undertook a PhD at the University of Aston in Birmingham. In 2005 he was appointed as Academic Fellow at The School of Pharmacy and became a lecturer in 2010. He has over hundread publications, including fifty journal articles, over fifty peer-reviewed abstracts and several international conference presentations. He also has six patents on vaccine formulations. His research focus is on designing, understanding & developing technologies for novel nanocarrier systems in overcoming biological barriers for the targeted delivery of small therapeutic molecules and macromolecules (proteins, peptides, siRNA, miRNA) via the pulmonary route in the treatment of lung diseases (lung cancer, asthma, COPD) and ocular conditions.
Abstract:
Fisetin or 3, 3’, 4’, 7-tetrahydroxyflavone is a natural flavonoid which can be found in different fruits and vegetables. Apart from its antioxidant, anti-inflammatory and neuroprotective activities, several studies have shown its anticancer effect in several cancer cell lines (e.g. lung, colon and prostate). This study explores the incorporation of fisetin into the cavity of different cyclodextrins to improve its poor aqueous solubility (< 1 mg/ml), that hinders its delivery. The complex was further engineered into an inhalable dry powder formulation that will potentially be useful to target the lung for therapeutic applications.
The highest complexation was found between fisetin and sulfobutylether-β-cyclodextrin (SBE-β-CD), and addition of 20%v/v ethanol increased the complexation by 5.9-fold. The spray-dried complex from the ethanolic solution showed an improved aerosolization performance, indicated by 2-fold increase in the fine particle fraction, compared to the spray-dried complex from aqueous solution. This may be caused by the lighter and less dense properties of the particles, showed by the pitted morphological surfaces, suggesting a hollower internal structure. Further incorporation of 20%w/w leucine improved the physical and aerosolization properties of the spray-dried complex. The preparation also showed an unchanged cytotoxic activity of fisetin against the human lung adenocarcinoma cell line (A549). In conclusion, the inhalable dry powder of fisetin-SBE-β-CD complex was able to increase aqueous solubility of fisetin and may be useful to deliver fisetin to the deep lung region.
- Smart Drug Delivery Systems | Vaccine Drug Delivery Systems | Routes of Drug Delivery
Location: London, UK
Session Introduction
Hyungil Jung
Yonsei University, South Korea
Title: Microneedle: the future of pharmaceutical and cosmeceutical delivery systems
Time : 12:30-12:50

Biography:
Hyungil Jung has completed his Ph.D. from Cornell University and his postdoctoral from California Institute of Technology (Caltech). Since then he had received various awards in biotechnology field such as “Outstanding Contributions”, “Best Contribution Award”, “Excellence in Research Award”, “The 31st Industry-academic Cooperation Award”, “Best technology Award”, “Best Teaching Award” and many more because of his outstanding research ability in biotechnology field. Dr. Jung has also recently registered his company, Juvic Inc. to further expand his research and to introduce novel microneedle based pharmaceutical and cosmeceutical products to the market.
Abstract:
Microneedles are microscopic needles capable of delivering pharmaceutical compounds, proteins and even cosmetics into the skin in a minimally invasive manner. There are various types of microneedles like solid, hollow and dissolving microneedle. Each of these microneedles, based on the application purposes can be applied in different branches of drug or cosmetic compounds delivery. Through microneedles, achievement of a highly efficient delivery has become possible and we expecting microneedles to replace the widely used hypodermic needles in near future. Rather than the drug delivery, microneedles can be applied in cosmetics field like anti-wrinkles, whitening or even anti-ageing. To develop a microneedle system that can fully replace hypodermic needles, we should focus on solving the limitations of current microneedle system like loading amount limitation, delivery time limitation, application limitation etc. In our laboratory at Yonsei University, and at Juvic Inc., we have already solved the main limitations of traditional microneedle systems through various patented technologies.
Steve Rannard
University of Liverpool, UK
Title: Solid drug nanoparticles as oral and long acting parenteral drug delivery for infectious diseases
Time : 12:50-13:10

Biography:
Steve Rannard is a materials chemist at the University of Liverpool (UoL) where he holds a personal Chair in the Department of Chemistry. He is the academic lead for Nanomedicine within the Materials Innovation Factory and Director of the Radiomaterials Laboratory. Steve spent 16 years in industry (Cookson, Courtaulds, Unilever) and has co-founded three start-up companies (IOTA NanoSolutions Ltd, Hydra Polymers Ltd and Tandem Nano Ltd). Since returning to academia in 2007 his collaborative grant income from funders including MRC (UK), EPSRC (UK), NIH (US), USAID, CRUK, CHAI, and BSAC (UK) and industry has exceeded £16m and his current research focuses materials science onto unmet medical/clinical needs to target new patient benefits using scalable polymer nanoparticle synthesis, solid drug nanoparticle formulation and nanoemulsion platforms.
Abstract:
Nanomedicine has focused heavily considerably on acute disease over several decades; however, there is considerable clinical need for new interventions for infectious disease prevention and therapy that allows patients to manage currently life-long conditions. Oral dosing is the only widely patient-acceptable administration format for chronic disease as daily, or more frequent, injections are not well tolerated. For prevention of disease and long term therapy, adherence to dosing regimens is critical to either maintain control or maintain protection over long periods. Here, we have generated a new approach to solid drug nanoparticle (SDN) formation and rapid candidate therapy identification that allows 1000’s of nanoparticle options to be generated and accelerated through a series of pharmacological tests to establish potential benefits. Two case studies will be presented, namely a candidate for reduced oral dose HIV therapy and a prophylactic antimalarial injection that provides long-term protection to infection. Methodology & Theoretical Orientation: Solid drug nanoparticle candidates were generated using an accelerated emulsion-templated freeze drying (ETFD) screening approach in both cases of HIV and malaria nanomedicine production. “Hits” were selected based on their chemical performance and progressed to a series of pharmacological studies that characterized a number of relevant factors. “Leads” were selected based on their pharmacological potential and, in the case of HIV candidate nanomedicines, translated from ETFD screen manufacture to cGMP production using emulsion spray drying (EFD). Powdered products from EFD were hand filled into capsules for human healthy volunteer evaluation. Findings: Oral dosing of two HIV antiretroviral SDNs has shown the potential for a 50% reduction of the dose of drug within daily regimens containing efavirenz or lopinavir. In the case of antimalarial prophylaxis, an intramuscular depot injection of SDNs has been shown to produce a minimum of 28 day protection in a mouse model, offering possible long-term protection in future human studies. Conclusion & Significance: Combined and systematic solid drug nanoparticle screening by both materials chemistry and pharmacology allows rapid identification of new nanomedicine candidates for diverse diseases with the potential for rapid translation to clinic.
Achim Aigner
Leipzig University, Germany
Title: Polymeric nanoparticles for therapeutic siRNA delivery: analysis of tissue-penetration and biological activities in tumor tissue slice cultures and in vivo xenograft models
Time : 14:00-14:20

Biography:
Achim Aigner is Professor for Clinical Pharmacology with research interests in the (preclinical) development of novel therapeutic strategies based on small RNA molecules (siRNAs, miRNAs, antimiRs) in oncology. One major focus is on the development and evaluation of polymer-based nanoparticles for in vivo use, including chemical modifications. Systems are tested in various in vitro, ex vivo and in vivo models of solid tumors. To this end, different target genes (established and novel oncogenes) as well as tumor inhibitory miRNAs or the inhibition of oncogenic miRNAs are explored.
Abstract:
The efficient delivery of small RNA molecules like siRNAs or miRNAs still represents a major hurdle in their therapeutic application for gene knockdown or miRNA replacement. Polymeric nanoparticles e.g. based on low molecular weight polyethyleneimines (PEIs) have been successfully explored, and chemical modifications further increase efficacy and improve biocompatibility. Among those, promising strategies include the modification of PEI with amino acids like tyrosine, yielding low molecular weight Tyr-PEIs (PxY with x = 2kDa, 5kDa, 10kDa), or the combination of PEI-based polyplexes with liposomes, resulting in lipopolyplexes. Both systems demonstrate improved in vitro properties and excellent applicability in vivo, as shown in mouse tumor xenograft models.
The therapeutic success of nanoparticles depends, among others, on their ability to penetrate a tissue for actually reaching the target cells, and their efficient cellular uptake in the context of intact tissue and stroma. Thus, beyond rather artificial tissue culture or rather tedious in vivo models, efficient ex vivo systems closely mimicking in vivo tissue properties are needed.
We have established tumor tissue slice cultures for the analysis of tissue-penetrating properties and biological activities of nanoparticles. As a model system, we employed slice cultures from different tumor xenograft tissues for analyzing modified or non-modified PEI/siRNA complexes and their lipopolyplex derivatives. Excellent tissue preservation was observed for >14 days, thus allowing for prolonged experimentation and analysis. Fluorescence microscopy of cryo-sectioned tissue slices shows different degrees of nanoparticle tissue penetration, dependent on their surface charge. More importantly, the determination of siRNA-mediated knockdown efficacies of endogenous target (onco-) genes reveals the possibility to accurately assess biological nanoparticle activities in situ, i.e. in living cells in their original environment. Thus, we introduce tumor (xenograft) tissue slices for the facile ex vivo assessment of important biological nanoparticle properties in a relevant setting.
Fiorenza Rancan
Universitätsmedizin Berlin, Germany
Title: Drug delivery across skin barrier: Investigation on different biocompatible thermoresponsive soft nanocarriers
Time : 14:20-14:40

Biography:
Fiorenza Rancan expertise lies in the use of human skin explants and skin organ culture as model for skin penetration and drug delivery studies. Within the last years she extensively investigated nanocarrier skin interactions with focus on both biological properties and toxicological effects of particle-based systems. Main research topics are the use of biodegradable particles (e.g. PLA and virus-like particles) for the delivery of adjuvants and antigens to skin (transcutaneous vaccination), the exploration of new generation nanocarriers for the treatment of skin inflammatory conditions giving special attention to antigen presenting cells, and the development of new antimicrobial treatments using skin models for infected chronic wounds.
Abstract:
Despite skin accessibility, delivery of drugs across skin barrier and the maintenance of a constant drug concentration in the target region is still a challenge. Nanocarrier-based approaches have been shown to improve both dermal and transdermal drug delivery. Depending on the type of nanocarrier, different drug release properties as well as different interactions with skin barriers and cell components can be achieved. A systematic correlation between nanocarrier physicochemical characteristics and their skin penetration and drug delivery properties is necessary to foster the use of nanotechnology in dermatology. Nanocarriers physicochemical properties and their performance after application on human skin explants have been investigated by methods like atomic force microscopy and stimulated Raman spectroscopy, whereas fluorescence and electron microscopy as well as flow cytometry of single skin cells served to elucidate nanocarrier penetration pathway and cellular uptake. Results show that size, surface charge, type of cargo, softness, and stability mostly influence nanocarrier penetration and drug delivery to skin. In particular, thermoresponsive nanogels which can release loaded drugs preferentially above a distinct temperature, represented an attractive approach to improve the selectivity of anti-inflammatory therapies. In addition soft, thermoresponsive nanogels were found to penetrate deeply within the stratum corneum, the outermost skin barrier, changing its permeability and improving drug penetration. Penetrated nanogels were internalized by skin cells in both epidermis and dermis. Interestingly, also significant percentage of antigen presenting cells were found to be associated with nanocarriers depending on the degree of skin barrier disruption. These observations could further be developed for specific targeting approaches in order to increase drug delivery to key cell populations.
Patrizia Chetoni
University of Pisa, Italy
Title: Investigation on nano-sized drug delivery systems for ocular application
Time : 15:00-15:20

Biography:
Patrizia Chetoni earned her PhD in Pharmaceutical Sciences in 1991 at the University of Pisa and she is currently Associate Professor of Pharmaceutical Technology at the Department of Pharmacy of the same University. She has conducted extensive researches in the technologies for drug delivery. In particular, she has worked in the development of strategies to improve absorption of drugs through biological barriers (cornea, skin, buccal mucosa and nail) and in the development of cell cultures models for prediction of drug bioavailability and of their cytotoxicity. She has also studied animal models to determine the bioavailability of topically applied drugs and experimental methods for the characterization of mucoadhesive properties of drug delivery systems. She has published more than 70 research papers in international referred journals she has also contributed to some book chapters and international patents.
Abstract:
The unique characteristics of the eye and the presence of strong defence mechanisms make difficult to achieve therapeutic concentrations of drug in the different districts of the eye after topical instillation of eye-drop. One of the main challenge to increase the poor ocular bioavailability of conventional formulations is to improve the low drug-contact time by reducing drainage, tear turnover and dilution or lacrimation. In addition, another strategy is to enhance the drug penetration across the cornea, which represents an effective barrier to drug permeation due to the presence of the annular tight junctions on corneal epithelium. Various drug delivery systems have been developed to increase the bioavailability of ophthalmic drug. In particular, nano-sized carriers like liposome, nanoparticle, SLN and nanomicelle have gained wide interest, providing an increase in the pre-corneal residence time, mucoadhesion and penetration across the eye tissues of drugs. Conventional delivery systems usually require administering at regular time intervals, whereas nano-sized carriers often release drugs at constant rate for a prolonged period of time and thus enhance their absorption and promote a site specific delivery especially when dispersed or suspended in polymer solutions with mucoadhesion properties. In fact, when applied topically as eye-drop, liposomes can attach to the hydrophobic corneal epithelium, where they continuously release the encapsulated drug, improving pharmacokinetics behaviour and decreasing toxic side effects of encapsulated drugs. Polymeric nanoparticles protect drug from metabolic degradation and interact strongly with both ocular surface and drug, more than the same mucoadhesive polymer in solution. SLN for their lipophilic nature, favour drug permeation through the highly hydrophobic corneal epithelium and furthermore, transscleral route might contribute to drug absorption in vitreous humour. The consistent progress in formulation efficiency of nano-sized carriers will be described in view of their application in ophthalmic field.
Balint Sinko
Pion Inc., USA
Title: Application of simultaneous dissolution-absorption apparatus for screening formulations before bioequivalence studies
Time : 15:00-15:20

Biography:
Bálint Sinkó received M.Sc. degree in Pharmacy at Semmelweis University in 2007. As a graduate student and a research fellow of Krisztina Takács-Novák his work focused on chemical analysis of Pharmaceutical formulations. He has started his Ph.D. in the same group in 2007 taking part in the installation of a new permeability lab. During his research he has developed a PAMPA model for the prediction of skin penetration that formed the main part of his Ph.D. thesis. He received his Ph.D. degree in 2012, while the developed Skin PAMPA model has been licensed to Pion Inc in the same year. After PhD he has joined Pion Inc. where he currently works as manager of technology development and support.
Abstract:
For generic drug development traditional dissolution tests have been used in the pharmaceutical industry to compare performance of different drug product formulations before conducting bioequivalence studies, even though the in vivo predictive power of these tests are questionable. When a poorly water-soluble API is formulated to enhance its dissolution, additives have an effect not only on dissolution, but also on flux through the membrane. The aim of this study was to demonstrate that a simultaneous dissolution-absorption test can be used as a predictive tool before bioequivalence studies are conducted. Telmisartan tablets were tested using MacroFLUX. Receiver chamber integrated with permeation membrane, overhead stirrer and UV probe was inserted in the standard 900 mL vessel of USP II apparatus.
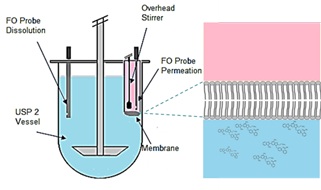
An artificial membrane with 3.8 cm2 area was separating the dissolution compartment from the receiver compartment containing 15 mL of pH7.4 buffer. The dissolution and flux results of the brand name (Micardis) and generic (Actavis) Telmisartan 40 mg tablets were compared. Actavis showed a slower release kinetics than Micardis, though reached the same maximum concentration after 110 min. The flux from the generic product was found to be 0.240 ± 0.011 µg/(cm2*min), which is only 71% of the flux of the brand name (0.337 ± 0.028 µg/(cm2*min)). This in vitro result showed excellent correlation with the in vivo data from bioequivalence studies, where the appearence rate or the drug in blood from Actavis was 72 % of the rate from Micardis. The in vivo predictive power of the simultaneous dissolution-absorption test was demonstrated by comparing the in vitro fluxes to in vivo rate of appearance in blood of brand name and generic formulation of telmisartan.
Christine Dufès
University of Strathclyde, UK
Title: Tumour regression after intravenous administration of novel tumour-targeted nanomedicines
Time : 15:20-15:40

Biography:
Christine Dufès is a Senior Lecturer at the Strathclyde Institute of Pharmacy and Biomedical Sciences (SIPBS), University of Strathclyde, Glasgow, United Kingdom. She obtained a Doctorate in Pharmacy (with Distinction and congratulations of the Jury) and a PhD (with a European Label, Distinction and congratulations of the Jury) from the University of Poitiers (France). After four years as a post-doctoral researcher at the Cancer Research UK Beatson Laboratories in Glasgow, she was appointed as a Lecturer at SIPBS in 2006, obtained fellowship of the Higher Education Academy in 2007 and became a Senior Lecturer in 2012. Her research interests include the targeted delivery of drugs and therapeutic genes to tumours and cerebral diseases. She has been awarded the Biochemical Journal Young Investigator Award (2009) and the Tom Gibson Memorial Award (2012) for her research, in addition to the Best Overall Strathclyde Teaching Excellence Award 2013 for her teaching. She sits on the editorial boards for 17 journals.
Abstract:
The possibility of using genes as medicines to treat cancer is limited by the lack of safe and efficacious delivery systems able to deliver therapeutic genes selectively to tumours by intravenous administration, without secondary effects to healthy tissues. In order to remediate to this problem, we investigated if the conjugation of the generation 3 diaminobutyric polypropylenimine dendrimer to transferrin and lactoferrin, whose receptors are overexpressed on numerous cancers, could result in a selective gene delivery to tumours after intravenous administration, leading to an increased therapeutic efficacy. The intravenous administration of transferrin-bearing and lactoferrin-bearing polypropylenimine dendriplexes resulted in gene expression mainly in the tumours. Consequently, the intravenous administration of the transferrin-bearing delivery system complexed to a therapeutic DNA encoding tumour necrosis factor (TNF)α led to 90% tumour suppression over one month on A431 epidermoid tumours. It also resulted in tumour suppression for 60% of PC-3 and 50% of DU145 prostate tumours. Furthermore, the intravenous administration of the lactoferrin-bearing targeted dendriplexes encoding TNFα led to the complete suppression of 60% of A431 tumours and up to 50% of B16-F10 skin tumours over one month. Transferrin- and lactoferrin-bearing polypropylenimine dendrimers are therefore highly promising delivery systems for cancer therapy.
- Nanotechnology in Drug Delivery | Pharmaceutical Nanotechnology | Biomaterials in Drug Delivery
Location: London, UK
Session Introduction
Flavia Laffleur
University of Innsbruck, Austria
Title: Biomaterials a pathway to overcome biomembranes
Time : 16:00-16:20

Biography:
Flavia Laffleur, is a senior researcher of Drug Delivery in the Department of Pharmacy at LFU Innsbruck, Austria. Flavia Laffleur published over 55 publications and gave oral presentations on several international conferences. From 2010 until 2013 she completed her doctoral thesis focused on smart drug delivery systems. Since 2013, she is a senior researcher at the Department of Pharmaceutical Technology in Innsbruck. Since 2017, Dr. Flavia Laffleur is a researcher at the MIT, in Boston, Massachusetts. She received several awards, including Lesmüller-Stiftung award and the Galenus Foundation Technology Award. Currently Dr. Laffleur´s research focusses on mucosal drug delivery as well as smart delivery systems to overcome biological barriers
Abstract:
Dry eye – a disease affecting between 4 and 34 % of the population worldwide. Stressful conditions to ocular surface, contact lenses, systemic disease (e.g. antidepressants, thyroid disease and diuretics cause dry eye. Complaints are dryness and tear film instability as well as evaporation caused by ocular surface changes. Therefore, it was aimed to investigate novel synthesized hyaluronic acid derivate evaluating its potential in mucoadhesion and lubricant for the treatment of dry eye syndrome. Hyaluronic acid, a well-known biomaterial in the ocular delivery was chemically modified with cysteine ethyl ester (HA-CYS). HA-CYS was evaluated in terms of mucoadhesive strength on ocular mucosa. Stability measurements and lubricative assay were conducted in form of disintegration and water uptake capacity, respectively. Moreover, safety consideration proceeded with in vitro cell line. Most important Hen's Egg Test on the chorionallantoic membrane for the mucous membrane compatibility was evaluated. According to the results HA-CYS achieved due to this thiolation more pronounced mucoadhesive, stability and lubricative properties enhanced. 3.81-fold increased swelling capacity, 30.5- fold more improved mucoadhesive properties and 9.72-fold higher stability of hyaluronic acid was achieved due to the chemical modification. Thus, the promising results underpin further exploitation of this versatile polysaccharide for treating dry eye syndrome
António José Ribeiro
University of Coimbra, Portugal
Title: Nanoprecipitation for delivery of Insulin
Time : 16:20-16:40

Biography:
Antonio Ribeiro is a Professor of Pharmaceutical Technology at the Faculty of Pharmacy of University of Coimbra where he managed a high international reputed research group. He has a Ph.D Degree in Pharmaceutical Development and Biopharmacy and his research has been focused on design of delivery systems for peptidic and protein drugs. He has published more than sixty peer-reviewed publications, among which several very highly cited, and he has been a keynote speaker and presented various talks all over the world. He is also an expert and consultant in intellectual property of drugs for pharmaceutical industry. He serves as an Editorial member of several publications and as a consultant for several research agencies mostly related to Diabetes and Nanotechnology.
Abstract:
Micro and nanoparticulates made of poly (lactic-co-glycolic acid) (PLGA) have been extensively studied due to their biocompatibility, biodegradability and ability to control release of drugs and have been linked to delivery of proteins. Nanoprecipitation is the most appropriate method to produce nano sizing particles for parenteral delivery without harsh conditions of temperature and agitation, making use of water miscible solvents and involving a simple set up. However, attempts to encapsulate proteins and specially insulin in PLGA nanoparticles using nanoprecipitation have revealed limited success due to a low efficiency of encapsulation /EE). Insulin-PLGA nanoparticles (Ins-PLGA NPs) for parenteral administration were produced by nanoprecipitation without any co-solvent or additive to insulin, buffering the dispersant phase. NPs were freeze-dried with sorbitol and characterized for size and polydispersity (PdI) and zeta potential. Insulin extracted from NPs was assayed using HPLC and its conformation was assessed before, during and after procedure using circular dichroism (CD). In vitro release studies were performed to access insulin release kinetics.
NP's with a mean size lower than 200 nm a low PDI were obtained after freeze-drying and revealed physical stability after reconstitution in water. EE of NP's was greatly improved compared to previous attempts, and among formulations, the choice of a buffer with a pH close to the PI of insulin revealed a higher EE. Insulin secondary conformation was maintained during manufacture so insulin was not markedly degraded during the manufacturing process. Insulin release from NP's showed a high burst effect and a release medium pH behavior. The PLGA based NP's buffered formation occurs under mild conditions and consequently can be used as a platform for delivery of labile molecules such as most of the biotechnology-based drugs.
Serena Mazzucchelli
University of Milan, Italy
Title: Metronomic treatment of breast cancer with Doxorubicin-loaded ferritin nanocages prevents chemoresistance and cardiotoxicity in comparison to liposomal Doxorubicin
Time : 16:40-17:00

Biography:
Serena Mazzucchelli, PhD, research associate at the University of Milan (UNIMI). Bachelor degree in Biological Sciences (2004), degree in Biology (2006) and PhD in Biological Sciences (2009) at the Department of Biotechnology and Biosciences (University of Milan-Bicocca-Italy). From 2009 to 2012 she has a post-doc fellowship at the Department of Biomedical and Clinical Sciences “L. Sacco” (DIBIC-UNIMI). Until 2015 she is researcher at the “L. Sacco” University Hospital. Today, SM is research associate of the Nanomedicine Laboratory carrying out her research focused on the development of nanodevices therapy of breast cancer at the DIBIC-UNIMI. She is an author of 35 papers and a reviewer.
Abstract:
Metronomic chemotherapy (LDM) is based on frequent drug administrations at lower doses, resulting in neovascularization inhibition and induction of tumor dormancy. LDM application in clinical practice is limited by: 1) low drug accumulation at tumor site, 2) controversial effectiveness against chemoresistance in advanced metastatic cancers, and 3) acquired resistance after prolonged treatment.
Nanotechnology could offer groundbreaking solutions to improve the effectiveness of LDM chemotherapy, by taking advantage of the unique targeting efficiency of ferritin (HFn) nanocages. Here, we exploit the HFn mediated targeted delivery of doxorubicin (DOX) in an aggressive breast cancer mouse model with DOX inducible chemoresistance.
HFnDOX was recently demonstrated to overcome chemoresistance by actively promoting DOX nuclear translocation in vitro and was tested as a MTD treatment on a DOX sensitive tumor model with encouraging results. We find that LDM administration of HFnDOX strongly improves the antitumor potential of DOX chemotherapy arresting the tumor progression, demonstrating that HFn mediate the nuclear delivery of DOX and increase its accumulation both in tumor tissue and in cancer cell nuclei. Moreover, we find that HFnDOX antitumor effect is attributable to multiple nanodrug actions beyond cell killing, including inhibition of tumor angiogenesis and avoidance of chemoresistance. Otherwise, although an even better reduction of tumor progression was achieved with liposomal DOX (plDOX), a fivefold increase in MDR1 positive cells has been displayed, suggesting that plDOX is not suitable in view of a protracted LDM treatment, due to the onset of chemoresistance. Multiparametric assessment of hearts, including histology, ultrastructural analysis of tissue morphology, and measurement of markers of reactive oxygen species and hepatic/renal conditions, provided evidence that metronomic HFnDOX allowed us to overcome cardiotoxicity contrary to what is observed with DOX and plDOX.
Our results suggest that HFnDOX has tremendous potential for the development of “nanometronomic” chemotherapy toward safe and tailored oncological treatments.
Victoria Sherwood
University of Dundee, UK
Title: An early developmental vertebrate model to assess nanomaterial safety
Time : 17:00-17:20

Biography:
Victoria Sherwood is a Discovery Fellow in Skin Cancer Biology at the University of Dundee. Dundee is ranked number one for biological research in the UK. Victoria’s research interests lie in how skin tumours progress into the most dangerous, metastatic forms of the disease and in the development of novel therapeutic strategies to treat these advanced tumours. This has led to an interest in my lab in developing targeted, theranostic NPs for the treatment of metastatic melanoma (one of the most aggressive forms of skin cancer). As part of this on-going work my lab have invested efforts in improving techniques to assess NP safety, as nanotoxicity is often a major barrier to the clinic for nanoformulations. Here I will present some of our published work to address these issues and will discuss future directions that we are taking in the lab to further improve safety assessment of nanotherapeutic materials.
Abstract:
Statement of the Problem: We are developing targeted, drug-loaded nanocarriers for treating the most aggressive forms of skin cancer (Baldelli Bombelli et al., 2014). These nanoformulations are complex, multicomponent drug delivery devices, which due to their high surface area-to-volume ratio and complexity in the materials used for construction, have the potential to result in toxicity when administered to patients. Thus, robust safety assessment is a major concern when developing such nanotherapeutic agents for the clinic.
Methodology & Theoretical Orientation: We have developed a system to rapidly and robustly assess nanoparticle (NP) safety, early on in the development process of novel nanotherapeutics (Webster et al., 2016; Al-Yousuf et al., 2017). This enables us to optimize the NP design and/or synthesis protocol in order to obtain nanotherapies that are safe for use in patients. Cell-based assays are the most commonly used approach for nanotoxicity assessment, but these methods are known to provide poor in vitro-in vivo correlations. We have incorporated an early developmental vertebrate phenotypic screening assay using Xenopus laevis as a model organism, into our nanotoxicity assessment protocol, to complement the cytotoxicity data. Combining data from these two nanotoxicity assessment approaches, provides an overall NP hazard assessment index. This index can inform researchers whether or not to progress with further assessment of NP safety in expensive, more labor/time intensive mammalian models, or to first refine the nanoformulation (Figure 1).
Findings: Using this approach, we assessed NP safety using a variety of nanomaterials (including formulations developed for biomedical applications). The approach could predict NP safety as confirmed through in vivo assessment in mice.
Conclusion & Significance: This work highlights the potential of early developmental models as a rapid screening tool for nanomaterial safety and suggests that such models could be incorporated into routine nanotoxicity assessment protocols.
Dimitrios A. Lamprou
University of Kent, UK
Title: Polymeric nanofibers for controlled release in hernia repair
Time : 17:20-17:40

Biography:
Dimitrios Lamprou (BEng, PgCert, PgDip, MSc, PhD, and MBA) is Associate Professor in Pharmaceutics & Drug Delivery at University of Kent (UK) and Visiting Academic at University of Strathclyde (UK). Lamprou has been trained in multidisciplinary areas, worked in first class laboratories, and has experience of teaching in Higher Education, conducting research and securing National and International Funding (over £1.5M). Dr Lamprou has authored over 50 articles in high impact multidisciplinary journals, and over 150 poster and podium presentations - that includes over 50 invited talks.
Abstract:
A hernia of the abdominal wall is a permanent or intermittent protrusion of abdominal contents outside the abdominal cavity through a defect in the abdominal wall. Hernia can be congenital or acquired, the latter mainly being as a result of the incision made during surgery. Many hernias can result in no symptoms, however some can go on to develop a range of problems from pain and cosmetic appearance to bowel obstruction, fistula and bowel ischaemia, which can be life-threatening. The majority of hernias are repaired by inserting a mesh to stabilise the weakness in the abdominal wall. These meshes can be biological (e.g. porcine) or synthetic. However, despite many different approaches to repair hernia with mesh, many hernias go on to recur or result in complications themselves (mesh erosion into surrounding organs; adhesions for example), increasing the potential symptoms and morbidities for the patient involved. The purposes of this study was to analysed the physical and physiological properties of an abdominal wall hernia and to develop a biological scaffold in order to overcome the physical and physiological limitations of abdominal wall hernia. Two advanced fabrication techniques (Electrospinning and 3D Printing) was used for the formulation of the scaffolds with and without drug molecules, in order to obtain a system that can facilitate hernia repair and wound healing. The systems was characterised by state-of-the-art techniques such as AFM, ToF-SIMS, CAG, Rheology.
Reno A L Leon
Structo Pte. Ltd., Singapore
Title: Multi-component orchestrated delivery modules for personalized healthcare using SLA 3D printing
Time : 17:40-18:00

Biography:
Reno A. L. Leon has completed his postdoctoral studies from National University of Singapore and is currently the CSO-Materials at Structo Pte. Ltd., Singapore. He has 4 years of industrial experience in polymeric materials for novel drug delivery, digital dentistry and micro-formulations. He has 2 patents and 5 peer reviewed publications to his credit. His research interests include 3D delivery systems, personalized healthcare solutions, 3D bioprinting and digital therapeutics.
Abstract:
Therapeutic delivery has long been the crux of medical advancement due to its direct affiliation with the patient. However the technological pathway hasn’t matched up with the growing demands of customizability and compliance. Unfortunately the present state of therapeutic prescription is at a standstill causing longer batch hours, huge stock piling, logistics cost, medication tracking and all this leading to enormous amounts of cost, man hours and compliance. Overarching all of the above is that the prescriptions are bulk manufactured with therapeutic amounts assigned based on averaged clinical data.
Here we report a first demonstration of customizable multi-modal delivery in tandem, ‘The Synco-Orchestration Delivery Module (SODM)’ using 3D printing. The objective of our work is to demonstrate an SLA 3D printed SODMs to address personalized drug delivery. Furthermore, SODMs are designed with multiple compartments to demonstrate multiple API delivery simultaneously. SODMs address most of the drawbacks of traditional delivery systems by bridging the gap between formulation, delivery and design. Significant advantages of SODMs include personalized dosage regimen, reduced number of intakes, programmed release kinetics and on-the-fly printable therapeutics. SODM thus promises a way to completely digitalize personalized delivery in the near future.
Dagmar Fischer
Friedrich-Schiller-University, Germany
Title: Bacterial nanocellulose as controlled drug delivery system in skin applications
Time : 11:50-12:30

Biography:
Dagmar Fischer, a pharmacist by training, has more than 20 years of experience in the field of nanocarriers based on synthetic and natural polymers, their formulation and biopharmaceutical characterization. Furthermore, she has long-standing successful cooperations with many partners in and outside of Europe, in the field of nanosafety. After receiving her PhD and Habilitation at the University of Marburg, she joined for several years a biotech company as Head of Preclinical Research and Development. In 2008 she was appointed as Professor of Pharmaceutical Technology at the University Jena.
Abstract:
The natural hydropolymer bacterial nanocellulose (BNC) is an innovative biomaterial, produced during fermentation by strains of Gram-negative bacteria Komagataeibacter xylinus and consisting of about 1% cellulose and 99% water. Although the chemical formula is identical to plant cellulose, the material favours totally different, but outstanding material characteristics due to the three-dimensional network of nano-sized fibres. The interest in BNC as drug delivery system dramatically increased during the last years, as the nano-sized 3D-network of BNC is expected to hold a large amount of drug molecules due to its large surface area. However, the highly hydrophilic character, limited a broad application, especially for the delivery of lipophilic drugs as well as long-term applications. We developed different loading techniques to accomplish a controlled release of drugs from several hours to weeks using BNC produced under lab-scale as well as under high throughput conditions. Native BNC, hybrid systems with different types of the thermo-responsive block-copolymers Poloxamers as well as lipid-modified BNC were established. Depending on the type of modification, not only the drug release profile, but also superior material properties such as high compression stability and water binding could be achieved. Using the antiseptic octenidine as model drug, the antimicrobial activity against Staphylococcus aureus and Pseudomonas aeruginosa was not changed by the use of the modified BNC. Excellent biocompatibility of the loaded BNC could be demonstrated after local administration in a shell-less hen’s egg model. In conclusion, controllable short- and long-term delivery systems consisting of Poloxamer and lipid modified BNC could be developed as ready-to-use systems e.g. for dermal wound treatment, cosmetics or the use as implants.
- Peptides and Protein Drug Delivery | Drug Targeting and Design | Nanoparticulate Drug Delivery Systems
Location: London, UK
Session Introduction
Bruno Sarmento
University of Porto, Portugal
Title: Nanoparticles-in-vaginal films for combined delivery of anti-HIV microbicide drugs
Time : 12:30-12:50

Biography:
Bruno Sarmento completed his PhD in Pharmaceutical Technology and Degree in Pharmaceutical Sciences at University of Porto, Portugal. He is an Affiliated Researcher at Institute of Investigation and Innovation in Health (i3S) and Institute of Biomedical Engineering (INEB), University of Porto, Portugal. He is an Assistant Professor of Pharmaceutical and Biopharmaceutical Technology at IUCS, Portugal. His current research is focused on “The development of functionalized nanomedicines and their application in the pharmaceutical and biomedical fields; in particular, nano-formulations of biopharmaceutical drugs with interest in diabetes, cancer and infectious diseases”. He has also specialization in “Mucosal tissue engineering models to validate functionalized nanomedicines and to perform in vitro/in vivo correlation”. He has published more than 160 papers in international peer reviewed (ISI) journals, 34 book chapters and more than 180 proceedings. He edited four books, participated in more than 50 invited/selected talks in national and international meetings and was awarded several distinctions. He is a member of Editorial Advisory Board of 10 international journals and has acted as referee for top-ranked journals in his area of expertise and for international funding agencies.
Abstract:
Statement of the Problem: There is an urgent need to reinforce battle against HIV/AIDS, namely by investing more in preventing new infections. Scientific and medical evidence produced over recent years supports that both oral and topical pre-exposure prophylaxis (PrEP) are promising approaches that can reduce sexual transmission of the virus. Still, anti-retroviral with different physicochemical properties may be challenging to combine in one single microbicide product.
Methodology & Theoretical Orientation: We propose a new system comprising the incorporation of nanoparticles (NPs) into films for the combined vaginal delivery of hydrophobic/hydrophilic molecules. EFV-loaded poly (lactic-co-glycolic acid) NPs were incorporated alongside free TFV into fast disintegrating films during film manufacturing. The delivery system was characterized for physicochemical properties, as well as for genital distribution, local and systemic 24 h pharmacokinetics, and safety upon intra vaginal administration to mice.
Results: EFV NPs with diameter of 145 nm were incorporated into a film with TFV. The film was soft and flexible. Disintegration time in simulated vaginal fluid was 9 min, resulting in dispersions with osmolality values near physiologic and pH of 4.24±0.02. The film presented low toxicity to CaSki, HEC-1-A and HeLa cells. NPs were evenly distributed and retained upon vaginal administration to mice. Mild epithelial penetration of NPs was observed. Drug concentrations in vaginal lavages and tissues peaked rapidly after film administration but slowly decreased up to 24 h. Still, drug concentrations were maintained at potentially protective levels. Films were found safe after daily 14 days administration as assessed by histological observation and analysis of IL-1b, IL-6, KC and TNFα levels.
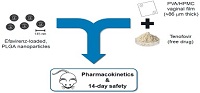


Conclusion & Significance: The proposed NPs-in-vaginal film is a promising new system that can adequately combine microbicide drugs with different solubility profiles. Results support that the system may be safe and able to provide sustained and potentially effective drug levels for preventing vaginal HIV transmission.
Imran Saleem
Liverpool John Moores University, UK
Title: Dry powder inhalation of Pneumococcal protein-based nanocarrier vaccine
Time : 12:50-13:10

Biography:
Imran Saleem is a Reader in Nanomedicine within the School of Pharmacy & Biomolecular Sciences, Liverpool John Moores University, UK. He has been working in and has published numerous papers in the area of pulmonary drug delivery since 2006. His research is aimed at developing novel delivery systems for targeting therapeutic agents to their site of action, with particular emphasis on lung diseases via dry powder pulmonary delivery. He has over 10 years’ experience in the area of nanoparticle formulation and drug delivery systems, and has published extensively in peer-reviewed journals, conference abstracts and book chapters.
Abstract:
There is a huge drive in the vaccine research field, pharmaceutical industry and Bill Gates Foundation for effective targeting of dendritic cells (DCs) to enhance the immune response and for needle-free vaccination. The aim of this study was to adsorb pneumococcal protein (PspA), onto poly(glycerol adipate-co-ω-pentadecalactone), PGA-co-PDL, nanoparticles (NPs) to target lung DCs. Further to formulate these NPs into dry powder nanocomposite microparticles (NCMPs) suitable for pulmonary vaccine delivery. NPs were prepared using an emulsion solvent evaporation method and PspA was adsorbed onto the surface of NPs (100: 20 [NP: PspA]). The NPs were spray-dried in an aqueous suspension of leucine (1:1.5) to produce NCMPs and characterised in terms of particle size, loading, cell viability, protein stability (SDS-PAGE), integrity (circular dichroism, CD), antigenicity (ELISA), immunization and aerosolisation studies. The NPs produced were 322.83 ± 4.25 nm in size with PspA loading 19.68 ± 2.74 µg/mg. The NCMPs resulted in a fine particle fraction (FPF%) >75%. The NPs appear to be well tolerated by DCs cell lines ≥90% cell viability) at 19.5µg/mL after 4h exposure. SDS-PAGE, CD (α-helical decreased < 13% vs standard PspA) and the antigenicity (>95%) confirmed that PspA was stable in both formulations after spray-drying. The cfu in BALF of mice challenged with pneumococcal bacteria was signifcantly less compared to PspA alone in the lungs or via subcutaneous injection. The PspA loaded NPs were incorporated into NCMPs having excellent aerosolisation characteristics whilst maintaining protein activity. Hence, it may be feasible to use these carriers for pulmonary vaccine delivery.
Marta Truffi
University of Milano, Italy
Title: Targeting active bowel inflammation foci by MAdCAM-1-specific nanoparticles
Time : 14:00-14:20

Biography:
Marta Truffi has her expertise in nano-biotechnology and cellular biology. Her current research is focused on the study of targeted nanosystems that will provide specific diagnosis and therapy of inflammatory bowel diseases. Her work involves in-depth study of the pathogenesis and identification of novel therapeutic targets, investigation of functional interactions between nanoparticles and cell cultures/tissues, development of in vitro and in vivo experimental models of human diseases, study of nanoparticles biodistribution and systemic toxicity. She is also interested in nanoparticles for breast cancer treatment, by coupling targeted delivery of chemotherapeutics with modulation of cancer-associated miRNAs, in order to improve patients’ responsiveness to therapy.
Abstract:
Statement of the Problem: Currently, the evaluation and treatment of inflammatory bowel disease (IBD) commonly relies on aspecific clinical signs of bowel inflammation, while specific targeted devices are still lacking. Mucosal addressin cell-adhesion molecule-1 (MAdCAM-1) has been proposed as a marker of bowel inflammation. It is upregulated on gut endothelium in IBD and is finely related to IBD activity and response to therapy. Here, we investigate a smart nano-platform targeted toward MAdCAM-1 for site-specific nano-theranostics in a preclinical model of IBD.
Methodology & Theoretical Orientation: We coupled anti-MAdCAM-1 antibodies to the surface of manganese oxide nanoparticles, and analyzed nanoconjugate biodistribution and safety in a murine model of IBD, by intravenous injection at the time of early acute phase of the disease.
Findings: Manganese oxide nanoparticles revealed good stability and negligible toxicity toward endothelial cell culture. Twenty-four hours post intravenous administration in colitic mice, fluorescent anti-MAdCAM-1-nanoparticles localized in the inflamed bowel, and specifically accumulated in the proximal part of the colon. By contrast, untargeted nanoparticles were more rapidly washed out. Nanoparticles did not induce histologic lesions in non-target organs.
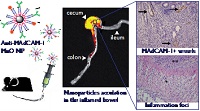
Conclusion & Significance: Anti-MAdCAM-1-nanoparticles uncovered active bowel inflammation foci, by following the expression pattern of MAdCAM-1 on mucosal vessels. The implementation of this nano-platform for early and specific theranostics applications appears promising for refining clinical care and management of IBD.
Satyanarayana Somavarapu
UCL School of Pharmacy, UK
Title: Formulation of inhaled phytochemicals
Time : 14:20-14:40

Biography:
Satyanarayana Somavarapu move to England came with the award of a Commonwealth Fellowship from the Association of Commonwealth Universities and he undertook a PhD at the University of Aston in Birmingham. In 2005 he was appointed as Academic Fellow at The School of Pharmacy and became a lecturer in 2010. He has over hundread publications, including fifty journal articles, over fifty peer-reviewed abstracts and several international conference presentations. He also has six patents on vaccine formulations. His research focus is on designing, understanding & developing technologies for novel nanocarrier systems in overcoming biological barriers for the targeted delivery of small therapeutic molecules and macromolecules (proteins, peptides, siRNA, miRNA) via the pulmonary route in the treatment of lung diseases (lung cancer, asthma, COPD) and ocular conditions.
Abstract:
Fisetin or 3, 3’, 4’, 7-tetrahydroxyflavone is a natural flavonoid which can be found in different fruits and vegetables. Apart from its antioxidant, anti-inflammatory and neuroprotective activities, several studies have shown its anticancer effect in several cancer cell lines (e.g. lung, colon and prostate). This study explores the incorporation of fisetin into the cavity of different cyclodextrins to improve its poor aqueous solubility (< 1 mg/ml), that hinders its delivery. The complex was further engineered into an inhalable dry powder formulation that will potentially be useful to target the lung for therapeutic applications.
The highest complexation was found between fisetin and sulfobutylether-β-cyclodextrin (SBE-β-CD), and addition of 20%v/v ethanol increased the complexation by 5.9-fold. The spray-dried complex from the ethanolic solution showed an improved aerosolization performance, indicated by 2-fold increase in the fine particle fraction, compared to the spray-dried complex from aqueous solution. This may be caused by the lighter and less dense properties of the particles, showed by the pitted morphological surfaces, suggesting a hollower internal structure. Further incorporation of 20%w/w leucine improved the physical and aerosolization properties of the spray-dried complex. The preparation also showed an unchanged cytotoxic activity of fisetin against the human lung adenocarcinoma cell line (A549). In conclusion, the inhalable dry powder of fisetin-SBE-β-CD complex was able to increase aqueous solubility of fisetin and may be useful to deliver fisetin to the deep lung region.
Khuloud Al Jamal
King’s College London, UK
Title: Double-targeting of glioma in mice using LRP1-targeting carbon nano-needles
Time : 14:40-15:00

Biography:
Khuloud T. Al-Jamal is a Chair of Drug Delivery & Nanomedicine at King’s College London (KCL). She was awarded the Overseas Research Award Scheme Scholarship from The University of London (2000-2004) to complete her PhD in Drug Delivery from The School of Pharmacy (currently known as UCL-School of Pharmacy). She was awarded the prestigious CW Maplethorpe Research and Teaching Postdoctoral Fellowship from The University of London (2005-2007) and started her academic career as a lecturer at KCL in 2011. She was awarded the prestigious Royal Pharmaceutical Society Science Award in 2012 in recognition for her outstanding scientific achievements in the field of Nanomedicine. She has developed an extensive experience in designing and developing novel nanoscale delivery systems including dendrimers, liposomes, quantum dots, polymers, viral vectors, chemically functionalised carbon nanotubes and graphene oxide. Her current work involves pre-clinical translation of novel nanomaterials designed specifically for drug, protein, nucleic acids and radionuclide delivery for therapeutic or diagnostic applications.
Abstract:
Statement of the Problem: Brain disorders are on the rise accounting for almost 12% of world mortalities every year. Despite extensive research in drug development, brain disorders are still largely untreated due to the inability to deliver current therapeutics to the brain across the BBB. Chemically functionalized carbon nanotubes (f-CNT) constitute a novel class of nanomaterials with attractive physical, chemical and electronic properties. One interesting characteristic of f-CNTs is their ability to translocate across plasma membranes and enter the cells either passively by direct translocation across membranes or actively via endocytosis. In this study, the brain uptake properties of multiwalled f-CNTs (f-MWNTs) were studied in in vitro and in vivo.
Methodology: An in vitro model consisting of PBEC and astrocytes were co-cultured in a Transwell™ system. Percentage of BBB crossing of radiolabelled [111In] DTPA-MWNTs was assessed at 37 °C up to 72 h or with an initial incubation at 4 °C for 4 h. Ultrathin sections of PBEC were imaged using electron microscopy. Brain uptake in vivo was evaluated by SPECT/CT imaging and gamma counting following intravenous injection of [111In] DTPA-MWNTs in mice.
Findings: The percentage transport of [111In] DTPA-MWNTs across PBEC (Figure A) increased over the course of 72 h. The initial 4 h-incubation at 4 â—¦C resulted in a slight but significantly lower % transport than that obtained at 37 â—¦C (P= 0.0005). This difference was abolished upon the re-incubation at 37 â—¦C at 72 h. The penetration process was captured by electron microscopy. The accumulation in mouse brain was confirmed by SPECT/CT imaging (Figure B). Superior brain uptake of ~2-5% ID/g was measured by gamma counting after whole body perfusion.
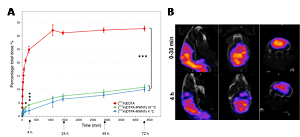
Conclusion & Significance: This is the first evidence of f-MWNTs translocation across the BBB. The significant reduction in BBB crossing at 4 °C confirmed the uptake was driven by an energy-dependent pathway. Electron micrographs revealed transcytosis of f-MWNTs and its sequence as a function of time. f-MWNT’s are able to access mice brain after i.v. injection.
Dimitrios A Lamprou
University of Kent, UK
Title: Metrology in pharmaceutical manufacturing
Time : 15:00-15:20

Biography:
Dimitrios Lamprou (Beng, PgCert, PgDip, MSc, PhD, MBA) is Associate Professor in Pharmaceutics appointed in September 2016 at University of Kent, and has been trained in multidisciplinary areas and worked in first class laboratories. Dr Lamprou has experience of teaching in Higher Education, conducting research and securing National and International funding (over £1M). Dr Lamprou has authored over 40 articles in high impact multidisciplinary journals, and over 130 poster and podium presentations at national and international conferences, that includes over 40 invited talks, and has experience of supervising Postdoc's, 20+ Ph.D students and 70+ Masters level research projects.
Abstract:
Structure-property relationships are often poorly defined in advanced continuous pharmaceutical manufacturing processes and products and hence it is difficult to control final product performance to the required degree to deliver advanced functionality. The dynamics of particles within complex mixtures and the effect of processes and storage on their disposition and microstructure is also challenging to measure. Hence, there is a clear need to have techniques for analysis and measurement of composition, dynamics and structure with increased spatial and temporal resolutions. Secondary Ion Mass Spectrometry (SIMS) is a technique that enables the analysis of the ionized particles (secondary ions) emitted when a surface is bombarded by energetic species (primary ions). To apply this technology to the study of Pharmaceutical Products is extremely interesting in order to investigate the precise distribution of Active Pharmaceutical Ingredients (APIs), impurities or excipients in the formulated product, also in a three dimensional visualization, to have a better understanding of the impact that this could have on its final performance. The aim of this work was to visualize the lateral and depth distribution of the drug in the polymer matrix, using ToF-SIMS, and to relate this data to the drug elution rate. The funduings was supported with data from other techniques, such as Atomic Force Microscopy (AFM), Raman Conmfocal Imagine, and microCT.
Wafa T. Al-Jamal
University of East Anglia, UK
Title: Targeted drug delivery to enhance cancer therapy and reduce its side effects
Time : 15:20-15:40

Biography:
Wafa Al-Jamal is an overseas and a UK-registered pharmacist. She completed her PhD in Drug Delivery in 2008 at UCL School of Pharmacy, London. She is currently a Prostate Cancer Research Fellow at The School of Pharmacy, University of East Anglia (UEA). She joined UEA as a Lecturer in Drug Delivery and Nanomedicine in 2013, after working as a senior research fellow at University College London and King’s College London. She was the GSK Emerging Scientist Award winner for 2015. Her main research interest focuses on engineering novel nanomaterials for biomedical applications. Her research has been funded by the Royal Society, Prostate Cancer UK, Research Council (EPSRC). She has published over 35 papers in high impact journals. Currently, she is a member the PCUK Research Advisory Committee and Visiting Professor at Guizhou Medical School, China.
Abstract:
Most cancer chemotherapeutics lack tissue specificity, resulting in many undesirable side effects. Delivering drugs selectively to the tumour tissues could ultimately increase local drug concentrations at the tumor without the need to escalate the administrated doses in patients. A wide range of drug delivery systems has been developed to alter the pharmacokinetics of the drug molecules and enhance their tumour targeting. Furthermore, several approaches have been explored to increase the bioavailability of drugs at the site of action, utilizing the unique characteristics of the tumor microenvironment, such as overexpressed enzymes, acidic pH, and hypoxia, or using external triggers, such as heat, ultrasound, and light. In this talk will describe the latest delivery systems that we have developed in our laboratory to enhance the tumour accumulation of anticancer drugs, utilising internal and external triggers.
Sharif Abdelghany
University of Jordan, Jordan
Title: PLGA nanoparticles entrapping amikacin and moxifloxacin as a potential host-directed agent therapy for multidrug resistant tuberculosis
Time : 15:40-16:00

Biography:
Sharif Abdelghany completed his PhD from Queen’s University Belfast, UK in drug delivery and Biomaterials in 2012. During his PhD, he developed a wide expertise in the formulation of targeted polymeric nanoparticles for cancer treatment and infectious diseases. After that, He joined the university of Jordan as an assistant professor in the department of Pharmaceutics and Pharmaceutical Technology where he developed polymeric nanoparticles formulation for the treatment of tuberculosis. He also received an endeavor research fellowship to conduct a short term fellowship for the dual nanoparticles entrapment of second line anti-TB drugs at the university of Sydney, Australia (April 2015-August 2015).
Abstract:
Polymeric nanoparticles have been widely investigated as a controlled release drug delivery platform for the treatment of tuberculosis (TB). These nanoparticles were also readily internalised into macrophages, leading to high intracellular drug concentration. In this study two anti-TB drugs, amikacin and moxifloxacin were encapsulated into PLGA nanoparticles. The novelty of this work appears in: (1) the efficient encapsulation of two hydrophilic second-line anti-TB drugs,and (2) intramacrophage delivery of this synergistic combination potentially for rapid treatment of multi-drug resistant TB (MDR-TB). Two water-oil-water (w/o/w) emulsion strategies were employed in this study: (1) alginate coated PLGA nanoparticles, and (2) alginate entrapped PLGA nanoparticles. The average particle size and polydispersity index (PDI) of the alginate coated PLGA nanoparticles were found to be unfavourably high with values of 640 ± 32 nm and 0.63 ± 0.09, respectively. In contrast, the alginate entrapped PLGA nanoparticles were within the desirable particle size range of 282 - 315 nm and the PDI was 0.08 - 0.16, and therefore were chosen for subsequent studies. Alginate entrapped PLGA nanoparticles yielded a drug loading of over 10 µg/mg powder for amikacin, and more than 5 µg/mg for moxifloxacin and entrapment efficiencies range of approximately 25-31% for moxifloxacin and 51-59% for amikacin. To study macrophage uptake efficiency, the nanoparticles of alginate entrapped nanoparticle formulation were loaded with acridine orange as a marker, seeded to THP-1 derived macrophages and viewed under confocal microscopy. The particles were readily internalised into the macrophages and highly concentrated in the nucleus region. Furthermore, the anti-mycobacterial activity of the drug-loaded particles was evaluated using M. tuberculosis-infected macrophages, which revealed a significant reduction (4 log reduction) of viable bacterial count compared to the untreated group. In conclusion, the amikacin-moxifloxacin alginate entrapped PLGA nanoparticles are promising for further in vivo studies. Particle internalisation of acridine orange loaded PLGA nanoparticles by THP-1 derived macrophages using confocal microscopy. The cells were treated with acridine orange loaded nanoparticles at two different concentrations of: (A) 100 µg/mL and (B) 10 µg/mL, and (C-D) PBS cells. (E) The fluorescence intensity of acridine orange loaded nanoparticles and DAPI with increasing Z dimension (corresponds to the intensity in different layers of the macrophage cell). The green color corresponds to the distribution of nanoparticles in the cytosol of THP-1 cells. The blue colour corresponds to the DAPI stained nucleus.
Poster Presentations/Exhibitions/Networking/B2B Meetings
Poster Presentations/Exhibitions/Networking/B2B Meetings
Title: Poster Presentations/Exhibitions/Networking/B2B Meetings
Biography:
Poster Sessions will be conducted in the Foyer Area...
Abstract:
For more details, PS: http://novel-drugdelivery-systems.pharmaceuticalconferences.com/poster-presentation.php
- Young Researchers Forum
Location: London, UK

Chair
Olivia Merkel
LMU Munchen, Germany

Co-Chair
Imran Saleem
Liverpool John Moores University, UK
Session Introduction
Marco Contardi
Italian Institute of Technology, Italy
Title: Transparent ciprofloxacin-polyvinylpyrrolidone antibiotic films and nanofiber mats as potential skin and wound care dressings
Time : 10:10-10:25

Biography:
Marco Contardi is a PhD student of Smart Materials group of the Nanophysics Department of Italian Institute of Technology in Genova. He obtained a Master’s degree in pharmaceutical chemistry and technology at the University of Palermo. After the graduation he began an internship period between Institute of Biophysics at National Council of Research of Palermo and Department of Physics and Chemistry of Palermo. Recently, he published 3 articles as co-author and his contribute regarded hydrogels preparation and their rheological and biological characterization and spectrofluorometric analysis of metformin.
Abstract:
Wound dressings have been evolving steadily in close conjunction with recent trends and advances in bio-based polymer processing. Dressings grew from materials or tapes that simply covered and concealed the wound, to materials that can interact with wounds so that important wound healing factors such as moisture management, active ingredient delivery and interaction with cells or proteins can be tuned or enhanced in situ. For chronic non-healing wounds or burns, for instance, one of the most important aspects is to prevent infection. As such, dressings should provide a continuous or quick release of the antiseptic agent at the wound surface to provide a long-lasting antimicrobial action in combination with maintenance of physiologically moist environment for healing. Most commercially available wound dressings are frequently opaque. Visual observation of the healing process is therefore prevented. Infection or other complications cannot be detected with opaque coverings. During inspection, dressing removal causes discomfort but also healing disruption, bleeding and granuloma formation. Transparent wound dressings are therefore highly desirable. Particularly, antiseptic loaded and adhesive free transparent wound dressings that can be easily absorbed by the wound are highly desirable. Hence, we demonstrate a facile water-based solvent casting process to incorporate a common insoluble antibiotic known as ciprofloxacin (Cipro) in its unmodified form in polyvinylpyrrolidone polymer using aqueous acetic acid solutions. Acetic acid enabled transparency, enhancement in antiseptic effect and softer films. Preliminary in vivo tests on C57BL/6J mice displayed good capacity to absorb exudate and dissolution rate of transparent antibiotic films on a model wound.
Fatma Demir Duman
Koc University, Turkey
Title: Development of cationic Ag2S NIR emitting QD’s as new generation of theranostic nanoparticles
Time : 10:25-10:40

Biography:
Fatma Demir Duman has her expertise in design, synthesis and characterization of near-IR emitting quantum dots and gold nanoparticles and their applications as optical imaging, gene and drug delivery agents and investigation of in vitro and in vivo effects of designed nanostructures.
Abstract:
Quantum dots are semiconducting nanocrystals with diameters between 2-10 nanometers. Strong signal brightness, resistance to photo bleaching, size-tunable emission and large absorption coefficient across a wide spectral range make them powerful agent against conventional organic fluorescence dyes used in bioimaging applications. In addition to their optical imaging ability, quantum dots have multifunctional properties such as functionalization with targeting ligands for site specific delivery and loading with therapeutic drugs, peptides or oligonucleotides for drug and gene delivery applications thanks to their high surface to volume ratio. However, most of the synthesized QD’s emit in the visible region and they contain heavy metals such as Cd, Pb or Hg, which have intrinsic toxicity to living organisms. Ag2S QDs developed in recent years are of great interest with their excellent biocompatibility and strong emission intensity in near infrared region (NIR) of optical spectrum, offering higher photon penetration depth, lower absorption and scattering of light by cellular components and lower auto-fluorescence in the living tissue compared to visible region. Yet, the Ag2S compositions reported are still limited to anionic and PEGylated ones. We have focused on the development of first cationic Ag2S QDs for combined action of optical imaging and gene therapy. Here, we will discuss the synthesis of
- Young Researchers Forum
Location: London, UK
Session Introduction
Marco Contardi
Italian Institute of Technology, Italy
Title: Transparent ciprofloxacin-polyvinylpyrrolidone antibiotic films and nanofiber mats as potential skin and wound care dressings

Biography:
Marco Contardi is a PhD student of Smart Materials group of the Nanophysics Department of Italian Institute of Technology in Genova. He obtained a Master’s degree in pharmaceutical chemistry and technology at the University of Palermo. After the graduation he began an internship period between Institute of Biophysics at National Council of Research of Palermo and Department of Physics and Chemistry of Palermo. Recently, he published 3 articles as co-author and his contribute regarded hydrogels preparation and their rheological and biological characterization and spectrofluorometric analysis of metformin.
Abstract:
Wound dressings have been evolving steadily in close conjunction with recent trends and advances in bio-based polymer processing. Dressings grew from materials or tapes that simply covered and concealed the wound, to materials that can interact with wounds so that important wound healing factors such as moisture management, active ingredient delivery and interaction with cells or proteins can be tuned or enhanced in situ. For chronic non-healing wounds or burns, for instance, one of the most important aspects is to prevent infection. As such, dressings should provide a continuous or quick release of the antiseptic agent at the wound surface to provide a long-lasting antimicrobial action in combination with maintenance of physiologically moist environment for healing. Most commercially available wound dressings are frequently opaque. Visual observation of the healing process is therefore prevented. Infection or other complications cannot be detected with opaque coverings. During inspection, dressing removal causes discomfort but also healing disruption, bleeding and granuloma formation. Transparent wound dressings are therefore highly desirable. Particularly, antiseptic loaded and adhesive free transparent wound dressings that can be easily absorbed by the wound are highly desirable. Hence, we demonstrate a facile water-based solvent casting process to incorporate a common insoluble antibiotic known as ciprofloxacin (Cipro) in its unmodified form in polyvinylpyrrolidone polymer using aqueous acetic acid solutions. Acetic acid enabled transparency, enhancement in antiseptic effect and softer films. Preliminary in vivo tests on C57BL/6J mice displayed good capacity to absorb exudate and dissolution rate of transparent antibiotic films on a model wound.
Waisudin Badri
Claude Bernard Lyon-1 University, France
Title: Elaboration of polymeric nanoparticles containing Nigella Sativa seed oil and Indomethacin for dermal application

Biography:
Waisudin Badri has received his B.Sc. in 2006. He obtained his master degree in the field of Pharmaceutical technology and Cosmetology from Claude Bernard Lyon 1 University, France (2014). He began his PhD in the field of Pharmaceutical Technology at Claude Bernard Lyon 1 University, France (2015). He has published up to now 7 manuscripts in international journals.
Abstract:
Mourad Sala
Claude Bernard Lyon-1 University, France
Title: DSD-Double Solvent Displacement method for liposomes and solid lipid nanoparticles (SLNs) preparation

Biography:
After obtaining his master degree in Pharmaceutical technology and Cosmetology at Claude Bernard Lyon 1 University (France), Mourad SALA has started a PhD course in Pharmaceutical Technology since 2014 in the Claude Bernard Lyon 1 University, France . Meanwhile, he is a pharmacy student enrolled in 4-year hospital pharmacy residency programme in a teaching hospital (Hospice Civils de Lyon) in Lyon. He has already published two papers in reputed internationl journals.
Abstract:
Bruno Sarmento
University of Porto, Portugal
Title: Nanoparticles-in-vaginal films for combined delivery of anti-HIV microbicide drugs
Time : 12:50-13:10

Biography:
PhD in Pharmaceutical Technology and degree in Pharmaceutical Sciences, University of Porto, Portugal; Affiliated Researcher at Institute of Investigation and Innovation in Health (i3S) and Institute of Biomedical Engineering (INEB), University of Porto, Portugal; Assistant Professor of Pharmaceutical and Biopharmaceutical Technology at IUCS, Gandra, Portugal. His current research is focused on the development of functionalized nanomedicines and their application in the pharmaceutical and biomedical fields. In particular, nanoformulations of biopharmaceutical drugs with interest in diabetes, cancer and infectious diseases. He has also specialized in mucosal tissue engineering models to validate functionalized nanomedicines and to perform in vitro/in vivo correlation. He published more than 160 papers in international peer reviewed (ISI) journals, most in top journals (Q1 in Pharmaceutical Sciences; Q1 in Nanoscience and Nanotechnology; total citations 3350; accumulated impact factor 627; H-index 29), 34 book chapters and more than 180 proceedings. He edited 4 books, participated in more than 50 invited/selected talks in national and international meetings and was awarded several distinctions. He is member of the Editorial Advisory Board of 10 international journals and has acted as referee for top-ranked journals in his area of expertise, and for international funding agencies.
Abstract:
Statement of the Problem: There is an urgent need to reinforce battle against HIV/AIDS, namely by investing more in preventing new infections. Scientific and medical evidence produced over recent years supports that both oral and topical pre-exposure prophylaxis (PrEP) are promising approaches that can reduce sexual transmission of the virus. Still, antiretrovirals with different physicochemical properties may be challenging to combine in one single microbicide product.
Methodology & Theoretical Orientation: We propose a new system comprising the incorporation of nanoparticles (NPs) into films for the combined vaginal delivery of hydrophobic/hydrophilic molecules. EFV-loaded poly(lactic-co-glycolic acid) NPs were incorporated alongside free TFV into fast desintegrating films during film manufacturing. The delivery system was characterized for physicochemical properties, as well as for genital distribution, local and systemic 24 h pharmacokinetics, and safety upon intravaginal administration to mice.
Findings: EFV NPs with diameter of 145 nm were incorporated into a film with TFV. The film was soft and flexible. Disintegration time in simulated vaginal fluid was 9 min, resulting in dispersions with osmolality values near physiologic and pH of 4.24±0.02. The film presented low toxicity to CaSki, HEC-1-A and HeLa cells. NPs were evenly distributed and retained upon vaginal administration to mice. Mild epithelial penetration of NPs was observed. Drug concentrations in vaginal lavages and tissues peaked rapidly after film administration but slowly decreased up to 24h. Still, drug concentrations were maintained at potentially protective levels. Films were found safe after once daily 14-day administration as assessed by histological observation and analysis of IL-1b, IL-6, KC and TNFα levels.
Conclusion & Significance: The proposed NPs-in-vaginal film is a promising new system that can adequately combine microbicide drugs with different solubility profiles. Results support that the system may be safe and able to provide sustained and potentially effective drug levels for preventing vaginal HIV transmission.
- Nanotechnology in Drug Delivery | Pharmaceutical Nanotechnology | Biomaterials in Drug Delivery
Location: London, UK
Session Introduction
António José Ribeiro
University of Coimbra, Portugal
Title: Nanoparticles for oral delivery of Insulin: Facts and safety concerns
Time : 11:00-11:20

Biography:
Antonio Ribeiro is a Professor of Pharmaceutical Technology at the Faculty of Pharmacy of University of Coimbra where he managed a high international reputed research group. He has a Ph.D Degree in Pharmaceutical Development and Biopharmacy and his research has been focused on design of delivery systems for peptidic and protein drugs. He has published more than sixty peer-reviewed publications, among which several very highly cited, and he has been a keynote speaker and presented various talks all over the world. He is also an expert and consultant in intellectual property of drugs for pharmaceutical industry. He serves as an Editorial member of several publications and as a consultant for several research agencies mostly related to Diabetes and Nanotechnology.
Abstract:
Nanotechnology-based approaches towards an oral delivery of macromolecules such as insulin are increasingly therapeutic approaches towards the prevention or treatment of diabetes. Nanoencapsulation of insulin increases its protection against enzymatic degradation, and facilitates its absorption through intestinal membranes. However, the intestinal absorption of insulin nanoparticles may be related to a stimulatory reaction induced by the local mucosa immune system. Intestinal epithelium is an immune privileged organ capable of mediating immune reactions either playing a local protective role or by triggering an inflammatory response to the presence of nanoparticles. Compromising safety of insulin delivered by nanoparticles, by inadequacy or insufficiency of studies, may lead to exacerbation of the inflammatory pathways conducive to unwanted local and other severe adverse effects. Therefore, it is imperative to include comprehensive safety assays in early pre-clinical studies in order to increase the representativeness of the results and strengthen the potential of oral delivery of insulin by means of nanoparticles. Herein, focus will be put on recent reports in oral delivery of insulin nanoparticles for diabetes as well a critical analysis of the safety studies supporting their pre-clinical development. The improvement of early safety assessment by transitioning to quantitative, nanoparticle composition-immune performance relationship studies in representative models will also be elucidated. Thereby, the role and importance of rational optimization in the development of “safe by design” insulin nanoparticles will be contextualized in the field of diabetes..
Maria Manuela Gasper
University of Lisboa, Portugal
Title: Therapeutic potential against metastatic melanoma of a copper-based aquaporin inhibitor nanoformulated
Time : 11:20-11:40

Biography:
Maria Manuela Gaspar has completed her PhD in 2005 in Pharmaceutical Technology at University of Lisbon and Post-doctoral studies at University of Dublin, Trinity College. She is a Researcher at Research Institute for Medicines, iMed.ULisboa, University of Lisbon. The area of research has been focused on “Design, development and biological evaluation of drug delivery systems for improving the therapeutic index of incorporated molecules in infectious, inflammatory and cancer animal models”. She is a Co-author of numerous patents, papers in peer-reviewed journals and book chapters.
Abstract:
Statement of Problem: Clinical and preclinical studies evidence the overexpression of aquaporin (AQPs) in a high number of cancers. In this sense, the development of selective AQP inhibitors represents an alternative therapeutic strategy for these pathologies. Metallodrugs of copper and gold are potential candidates to fulfill this need. However, to promote a preferential target to tumor sites in vivo, the design and development of novel technologies based on nanostructured materials such as liposomes acting mainly as vectors for metallodrugs delivery represents a stimulating research area. Cuphen, a potent copper-based aquaporin inhibitor, was selected as metallodrug in the present work to be nanoformulated in liposomes.
Methodology: Cuphen was incorporated in liposomes by the dehydration-rehydration method. Cuphen liposomes were characterized in terms of mean size, poly dispersion index, zeta potential and encapsulation parameters using different lipid compositions. The ability of Cuphen to induce cancer cell death was evaluated by MTS and ViaCount assays against several human and mouse cell lines (A431, MNT-1, HaCaT, B16F10 and C26). The hemolytic activity of Cuphen in free and liposomal forms using EDTA-preserved peripheral human blood from voluntary donors was carried out. In vivo toxicity studies were performed in healthy mice and the therapeutic effect was evaluated in a murine melanoma xenograft model.
Findings: In vitro studies illustrated the anti-proliferative effects of Cuphen in different cancer cell lines. In vivo studies revealed no toxic effects after parenteral administration in healthy mice. A higher anti-cancer effect in a murine melanoma xenograft model in terms of survival rate was observed for the metallodrug nanoformulated in long circulating liposomes.
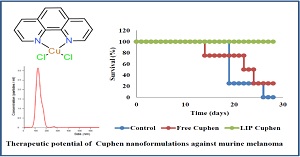
Conclusion: Cuphen liposomes are considered as a very attractive nano-formulation with therapeutic potential against melanoma due to their preferential extravasation and accumulation in solid tumors.
Anselmo J. Otero-Gonzalez
Havana University, Cuba
Title: Cm-p5: an antifungal mollusc-derived peptide conjugated with citric acid-modified manganese-ferrite nanoparticles with enhanced activity against Candida albicans in vitro
Time : 11:40-12:00

Biography:
Anselmo J. Otero-Gonzalez, Microbiologist, Havana University, 1978, PhD, National Centre for Scientific Research, Havana, 1987, Doctor in Science, Havana University, 2008, now is Senior Researcher, Antimicrobial Peptide lab, Faculty of Biology, Havana University, Cuba and president of National Board for PhD examination in Cuba. He is professor of Cell Engineering and Immunology. 1981: awarded with a fellowship for attending the Uppsala Separation School, Biomedical Centre University of Uppsala, Sweden. 1983: doctoral stay at the Department of Genetics, Pennsylvania University, Philadelphia, USA for monoclonal antibody generation. 1991: post-doctoral stay regarding Cell banking at the European Collection of Animal Cell Cultures, Porton Down, Salisbury. 1992: fellowship for a research project (AIDS-HIV vaccine), Swedish Centre of Disease Control, Stockholm, Sweden. 2000: awarded with a postdoctoral fellowship, David Rockefeller Centre of Latin-American Studies, Harvard School of Public Health, Harvard University, Boston, USA. 2011-12: post-doctoral stay at Harvard Medical School for characterizing the antifungal peptide Cm-p5. He also has (2008-present) collaboration with the Bioorganic Department, Leibniz Institute for Plant Biochemistry, Halle (Saale), Germany regarding antimicrobial peptide isolation, evaluation and characterization. Dr. Otero has more than 90 scientific articles in recognized journals and more that 145 abstract and presentations in scientific events.
Abstract:
Antimicrobial peptides form part of the first line of defence against pathogens for many organisms. Current treatments for fungal infections are limited by drug toxicity and pathogen resistance. Cm-p5 (SRSELIVHQRLF), a peptide derived from the marine mollusc Cenchritis muricatus (Gastropoda: Littorinidae) has a significantly increased fungistatic activity against pathogenic Candida albicans, exhibiting low toxic effects against a cultured mammalian cell line. Cm-p5 as characterized by Polarimetry of Circular Dichroism and Spectroscopy of Nuclear Magnetic Resonance. The antimicrobial activity of different types of nanoparticles has been previously demonstrated. Specifically, magnetic nanoparticles have been widely studied in biomedicine due to their physicochemical properties. The citric acid-modified manganese ferrite nanoparticles used in this study were characterized by high-resolution transmission electron microscopy, which confirmed the formation of nanocrystals of approximately 5 nm diameter. These nanoparticles were able to inhibit Candida albicans growth in vitro. However, the nanoparticles were not capable of inhibiting Gram-negative bacteria Escherichia coli or Gram-positive bacteria Staphylococcus aureus. The antifungal peptide Cm-p5 was conjugated to the modified manganese ferrite nanoparticles. The antifungal activity of the conjugated nanoparticles was higher than their bulk counterparts. This conjugate proved to be nontoxic to a macrophage cell line at concentrations that showed antimicrobial activity.
Pranav Shah
Uka Tarsadia University, India
Title: Asenapine Maleate loaded solid lipid nanoparticles for oral delivery
Time : 12:00-12:20

Biography:
Pranav Shah has completed his PhD from Maharaja Sayajirao University of Baroda, India. He is an Associate Professor at Maliba Pharmacy College, Gujarat, India. He has published 16 papers in reputed journals, two book chapters and one book. He has presented several papers in national and international conferences.
Abstract:
Asenapine maleate (ASM) is a new second-generation antipsychotic approved in August 2009 by U.S FDA for the acute treatment of schizophrenia and manic or mixed episodes associated with bipolar disorder in adults. It shows poor oral bioavailability of <2% due to extensive first pass metabolism in liver. The present study was aimed at developing and characterizing solid lipid nanoparticles (SLNs) of ASM. SLNs were prepared by solvent injection method by employing compritol ATO 888 as the lipid matrix and poloxamer 188 as stabilizer. A 32 full factorial design was employed to study the influence of independent variables (amount of lipid and % surfactant) on dependent variables (particle size, and % entrapment efficiency). Optimized ASM-loaded SLNs were further studied for zeta potential, in vitro drug release and TEM. Nanoparticles were lyophilized to improve the physical stability and obtain free flowing powder. Mannitol was employed as a cryoprotectant. Lyophilized ASM-loaded SLNs were characterized using DSC and XRD. The optimized ASM-loaded SLNs exhibited mean particle size 318.5±3.2 nm; polydispersity index of 0.255; zeta potential -29.75±(-0.92) mV; entrapment efficiency 53.13±1.77%; drug release extended up to 36 hours. TEM image exhibited spherical smooth surfaced nanoparticles. Accelerated stability studies of optimized ASM-loaded SLNs and lyophilized ASM-loaded SLNs revealed its stability. The developed formulation holds promising future due to reduction in dose and dosing frequency and thus reduces dose related side effects and improved patient compliance.
Muhammad Hanif
Bahauddin Zakariya University, Pakistan
Title: Thiolated pectin conatining hydrogels for controlled drug delivery of citrizine hydrochloride with its in-vitro studies
Time : 12:20-12:40

Biography:
Muhammad Hanif has completed his PhD from University of Karachi, Pakistan. He is the Managing Editor of Pakistan Journal of Pharmaceutical Research, a biannually published journal. He has published more than 45 papers in reputed journals and has supervised 15 MPhil and six PhD students. His speciality areas are “Formulation development of controlled release dosage forms, micro-encapsulations, hydrogels, in vitro and in vivo correlations (IVIVC), pharmacokinetic and stability studies.
Abstract:
Use of pectin in controlled release dosage form is reported in the previous literature and many dosage forms like tablets, microspheres and hydrogels were developed. Some studies reported the mucoadhesive properties of the pectin when used as drug career. In our study, we have developed thiolated pectin first by adding the thiol containing substances and then converted it into the hydrogel, having the capacity to use both lipophilic and hydrophilic drugs. Central composite rotatable design was successfully applied with the three variables and nine hydrogel formulations were planned in such a way that each three contains the difference in concentration of polymer, monomer and cross linking agent. Prepared formulations were further subjected to swelling, porosity, sol-gel fraction, cross-linked density and drug loading studies. Drug release studies were performed in USP 2 dissolution apparatus having the volume 900 ml at 370C and with 100 rpm rotation. In vitro kinetic model dependent and model independent approaches were applied to select the one best hydrogel formulation. Morphological studies like SEM, FTIR, TGA, DSC and XRD were performed to check any interaction between the polymer and drug. Prepared hydrogels were dried at room temperature first and then in oven at 450C. Maximum swelling observed was pH dependent and at 7.5 pH all formulations showed more than 100% swelling due to the presence of carboxylic acid group in the acrylic acid which has the pKa value four. Mucoadhesion in the thiolated pectin was increased as we increased the concentration of thiol glycolic acid. More than 85% release after 48 hours studies was found to be within limits. First order drug release was observed with the fickian diffusion because the n value was less than 0.45. Similar values proved that F5 formulation was the best due to its release pattern.
Fanzhu Li
Zhejiang Chinese Medical University, China
Title: A novel doxorubicin loaded folicacid conjugated PAMAM modified with borneol, a nature dual-functional product to freducing PAMAM toxicity and boosting BBB penetration
Time : 12:40-13:00

Biography:
Fanzhu Li, male, doctor, professor, doctoral supervisor and postdoctoral cooperative tutor. In march 2002, Prof. Li was introduced to Zhejiang Chinese Medical University as a special talent. Now, he is the dean of college of pharmaceutical science. Prof. Li engaged in novel drug delivery system, targeting preparation, new dosage form and novel technique as well as the process of chinese medicine in vivo for a long time. At the same time, Prof. Li has established targeted drug delivery systems of brain, liver, kidney and other organs, studied new methods of administration of nasal mucosa, and first initiated a new model of the process of traditional Chinese medicine in vivo in china.
Abstract:
E​ffective targeting drug delivery system for glioma treatment is still greatly challenged by the existence of the blood-brain barrier (BBB) and the intracranial over spreading of anti-tumor drug. Herein, we presented a dual-functional glioma targeting delivery of doxorubicin based on the PAMAMG5 dendrimer, modified with folic acid (FA) to target tumor cell, alsoborneol(BO), a well known safe material derived from traditional Chinese medicine, to facilitate the BBB permeability and reduce the toxicity of naked PAMAM. The intracranial transportation and glioma targeting ability were evaluated on the BBB model and C6 glioma cells in vitro. Also, pharmacokinetics and biodistribution were studied on C6 glioma-bearing rats in vivo. It indeed reduced the cytotoxicity of PAMAM against both HBMEC and C6 cells by coupling BO on the surface, while efficiently boosted BBB permeability with the improvement of transportation ratio by 2 folds to the BO-unmodified conjugates. Furthermore, conjugated FA increased total uptake amount by C6 cells leading to strong inhibition with the 3-fold lower IC50 value than FA-unmodified DOX conjugate. In comparison with DOX solution, FA-BO-PAMAM/DOX exhibited significantly prolonged half-life time and increased area under the curve and improved DOX accumulation in brain tumor. The tumor growth inhibition, in vivo, was significantly increased up to 57.4%. The median survival time of xenograft rats after administering FA-BO-PAMAM/DOX (28 days) was significantly prolonged compared to free DOX (18 days, P < 0.05) or other controls. In conclusion, this strategy of novel targeting nanocarrier provides apromising method to increase the drug accumulation in the tumor site for therapy of glioma.
Zaheer Ahmad
Chinese Academy of Sciences, China
Title: Hypoxia-responsive methoxy poly (ethylene glycol)-block-poly (glutamic acid)-graft-6-(2-nitroimidazole) hexyl amine nanoparticles for doxorubicin drug delivery
Time : 13:00-13:20

Biography:
Zaheer Ahmad completed his PhD at Quaid-I-Azam University Islamabad, Pakistan in 2016. He performed most of his PhD research abroad at University of Alberta, Canada (under the supervision of Professor Mike Serpe) and Changchun Institute of Applied Chemistry (under the supervision of Professor Xuesi Chen). His research interest focuses on “Development of pH and hypoxia responsive poly amino acid based nanocarriers and its in vitro and in vivo investigation using breast cancer mice xenograft model. He published about seven peer-reviewed papers in international journals such as Journal of Controlled Release, RSC Advances and Macromolecular Biosciences.
Abstract:
Tumor micro environment-targeted nano drug delivery vehicles are gaining mounting attention in the field of biomedical sciences. The hypoxic response of the tumorous cells due to very low partial pressure of oxygen (sometimes less than 2.5 mm of Hg) in the tumor tissues makes hypoxia responsive drug delivery system as the more appealing in cancer chemotherapy. Based on these considerations, we synthesized hypoxia responsive polymeric materials methoxy poly(ethylene glycol)-block-poly(glutamic acid)-graft-6-(2-nitroimidazole) hexyl amine (mPEG-b-PLG-g-NID) by conjugation of the hydrophobic nitroimidazole derivative (NID) [6-(2-nitroimidazole) hexyl amine] with the pendant carboxylic group of poly(ethylene glycol)-block-poly(L-glutamic acid) (mPEG-b-PLG). The structure and degree of substitution were confirmed by proton NMR, FTIR and UV-Vis spectroscopy. The degree of substitution was found to enhance with the increase in nitroimidazole derivative to polymer ratio. The hypoxia response of the material was evaluated by UV-Vis spectroscopy and zeta potential measurements. Doxorubicin was hydrophobically encapsulated in the micellar core of the hypoxia responsive nanoparticles. The drug loaded micelles showed faster release in hypoxic condition as compared to normoxic conditions. Moreover, the developed polymeric system was found non-toxic to MCF-7 cell line thus suggesting its biocompatibility and suitability as drug delivery device.

Biography:
Mohammad Salhab is an Academic Clinical Fellow in Orthopaedics at the unit with a PhD studentship in pharmaceutics and currently involved in developing the NM. Mr Salhab has published many articles in surgical field and also bioethics and basic science.
Abstract:
Background: Acute pain control following elective primary total knee replacements (TKRs) and total hip replacements (THRs) is often poor and is associated with long term chronic pain syndrome. Moderate to severe pain is often reported in the first 48 hours following surgery requiring different pain modality management strategies such as patient controlled analgesia and multimodal drug analgesia. The Local Infiltration Anaesthetic (LIA) technique is currently an established technique to tackle perioperative pain relief; however, studies have reported conflicting evidence so far. In a recent review of 29 studies investigating the use of LIA in TKR, LIA emerged as a safe technique with improved pain control (Gibbs DMR 2012). We have developed the LIA technique to include an intra-articular catheter allowing an infusion of Novel Mixture (NM) to be infused continuously postoperatively.
Kuldeep H Ramteke
Modern College of Pharmacy, India
Title: Various potentials of isolated bael fruit gum in drotaverine hydrochloride tablets

Biography:
Kuldeep Ramteke has his expertise in the application of newly accepted optimization technique i.e. SeDeM and he is having the good practical hand regarding the isolation of the natural gums from the natural sources. The main emphasize is on the application of the isolated gum for the development of the new drug delivery system or new use of the natural polymer.
Abstract:
Back ground: Gum constitute a major group of naturally occurring polymer, their abundance and low cost make them the preferred choice in foods and pharmaceuticals. Bael (Aegle Marmellose linn) fruits are edible, and reported in ancient system of medicines for their various activities.
Aim: The present research work was conducted to evaluate the various excipients potential of bale fruit gum (BFG) for solid dosage form. Material and Methods: BFG was isolated from partially ripe fruits of Bael. Identification and Standardization of BFG was carried out as per the stated procedures in the pharmacopoeia. Studies were conducted to support the physicochemical properties of gum. Solid state characterization of BFG was done by SEM, FTIR, DSC and XRD techniques. Different formulations were prepared to check the excipients profile of BFG like disintegrant, diluents, binder, carrier for solid dispersion, phosphorylated derivative and combination with other natural superdisintegrant and lyophilized derivative. Drotaverine Hydrochloride (DTV) was used as model drug for this experiment. Different tablet formulations were prepared and evaluated for physicochemical properties like appearance, average weight, hardness, thickness, disintegration, assay and dissolution. Prepared formulations were optimized by using the SeDeM diagram expert system to check the direct compression suitability of the blend.
Result: It was found that BFG possess good compressibility, flowability, low moisture content, neutral pH, amorphous nature and good water solubility. This made it suitable to be used as the release modulator in various dosage forms. It also possesses the binder and disintegrant property. When BFG was lyophilized, its surface characteristics were changed. Increase in pore size and ultimately the porosity and decrease in particle size proved its use as the superdisintegrant in immediate release tablets. Comparison of the BFG and Lyophilized BFG (LBFG) physicochemical properties shows that LBFG possess superior disintegrant potential compare to BFG for immediate Release tablet.
Conclusion: From the research work it was concluded that LBFG in concentration range of 10-12% shows superdisintegrant activity.
Muyiwa Arisekola
Walter Sisulu University, South Africa
Title: Anti-inflammatory activity of the volatile oil of the pulp of Raphia australis

Biography:
Muyiwa Arisekola is an emerging student researcher. He finished his 5-year Bachelor of Technology degree in pure and applied chemistry with a second class (upper division). His vision is to parlay his passion for organic chemistry into a drug design and discovery career via a profound understanding of pathogenesis of diseases and structure-activity relationship of drugs. He is currently pursuing a degree of masters of science (by research) in organic(medicinal natural products) chemistry. He is preparing his first paper for publication.
Abstract:
Statement of the Problem: Corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs) have been used for decades in the treatment of inflammatory disorders. Corticosteroids have limitations some of which are depression, glucose intolerance, and susceptibility to infection due to depressed body immunity. NSAIDs also have their side effects due to the undesired depletion of COX-1. After the development of COX-2 specific inhibitors, researchers later discovered that COX-2 also is constitutively produced in the body. The limitations of these NSAIDs have necessitated a research into natural products which show potent anti-inflammatory activity while at the same time do not interfere with the actions of COX-1 and COX-2 which are expressed in some specialized body tissues. The purpose of this study is to look into the use of volatile oil of Raphia australis as an anti-inflammatory agent . Methodology & Theoretical Orientation: The volatile oil of Raphia australis was extracted in hexane using Clevenger Apparatus, after which the GC-MS analysis of the oil was carried out. The anti-inflammatory test was done using four groups (1, 2, 3, and 4) of rats comprising six rats per group. Group one were administered normal saline (0.09% v/v NaCl) while group1,2 and 3 were administered 40mg/kg(oil), 80mg/kg(oil) and 100mg/kg aspirin respectively. Inflammation was induced in all the groups using lipopolysaccharide thirty minutes after treatment by gavage. The major constituents obtained from the GC-MS analysis of the volatile oil are: n-hexadecanoic acid (34.0%), tetradecanoic acid (9.5%), terpinolone (9.1%), dodecanoic acid (9.6%), hotrienol (8.8%), alpha-terpine-1,4-diene (.8%), transbenzalacetone (8.2%), nonanoic acid (7.7%) and beta-ionone (6.0%), lauric acid (9.6%). Conclusion and Significance: The 80mg/kg dose of the oil showed a significant anti-inflammatory effect (P<0.01) when compared to 100mg/kg of aspirin (P<0.01). The analgesic activities of aspirin (P<0.05) and the 80mg/kg dose (P<0.05) of the oil were only significant in the inflammatory phase of the analgesic test.

Biography:
To be updated...
Abstract:
Motivation:-Cancer is a group of diseases involving abnormal cell growth with the potential to spread to other parts of the body. Chemotherapy is a type of cancer treatment that uses drugs to destroy cancer cells. Thus realizing the need to overcome complexities involved in treating complex diseases motivated the author to design casein coated iron oxide nanoparticles (CCIONPs) crosslinked with glutaraldehyde for achieving efficient MDT. Method: - In order to design casein nanoparticles (CCIONPs) the microemulsion method was adopted and for impregnation of iron oxide nanoparticles into the bulk of casein matrix an in situ co precipitation method was used. In order to characterize nanoparticles FTIR, XPS, TEM, SEM, VSM, Mossbauer, zeta potential, in-vitro cytotoxicity test were studied. In order to investigate the drug release behaviour of drug loaded CCIONPs the magnetic fields of strength 1000G, 1500G, 2000G, 3000G, 5000G and 5500G were applied. The nanoparticles were loaded with cytarabine and its controlled release was investigated drug loading, chemical architecture of the nanocarriers, and nature of release media. In vivo analysis was perfomed on mice model and different cell lines. Results: - FTIR analysis confirmed homogenous deposition of iron oxide and subsequent formation of CCIONPs. The drug loading efficiency of CCIONPs, drug content and in vitro drug release profiles may be measured by ultraviolet spectrophotometer at λmax 254. It was found to have better payload, in vitro release profile characteristic and better targeting to RES organs. Conclusion: - Glutaraldehyde crosslinked casein coated iron oxide nanoparticles CCIONPs form a swelling controlled drug release system, which effectively delivers cytarabine in the presence and absence of magnetic field via diffusion controlled pathway. Thus, the prepared nanoparticles showed potential to provide a possible option for magnetically targeted delivery of anticancer drugs.
Tesfaye Zerihun
Addis Ababa University, Ethiopia
Title: Methalonic extract of the exudate of Aloe otallensis and its effect on Leishmania Eathiopica Parasite and its phytochemical screening

Biography:
I am working as a Senior Clinical Pharmacist at Addis Ababa University, college of Health Science, Black lion specialized Teaching Hospital In this teaching Hospital I am serving as Drug Supply Management coordinator, The Head of special Pharmacy of the hospital, the secretary of DTC (Drug therapeutic Committee) and other committee works. In addition to these Mentoring under graduate pharmacy students who are coming to the hospital for clinical attachment both at the ward and dispensary area .I am also participating in some of Clinical research which is under go in the Hospital beside the routine work.
Abstract:
Background & objectives: Several plant products have been tested and found to possess antileishmanial activity. The present study was undertaken to evaluate antileishmanial activity of methanolic extract of Aloe otallensis on the promastigot stage of Leishmania aethiopica comparing to standard drugs and also tried to screen its phytochemical constitute.
Methods: Phytochemical screening was done using the method mentioned by Evan and Trease on methanolic extract exudates of Aloe otallensis leaf. The extract was also evaluated for in vitro antileishmanial activity against Leishmania Aethiopica which is found from the black lion hospital parasitology unit. The result was compared to standard drug of Sodium stibogluconate, milfostin and paramomycin.
Result: The extract has a good antileishmaniacidal activity with an IC50 of 0.041 μg/ml on L. aethiopica (LDC/134). The experimental data shows that relatively it has better activity than paramomycin and milfostin but less activity than sodium stibogluconate. The data analyses was done by pad graph prison version 5 software after it was read by ELISA reader at the wave length of 650 nm. The phytochemical screening of the exudates of aloe otallensis showed the presence of phenol, alkaloid and saponin.
Conclusion: The methanol extract of exudate of Aloe otallensis has a good anti leishmanisis activity and this may be attributed to phenol, alkaloid and saponin presnt in the plant. But it needs further analysis for the conformation of which constituent present in much concentration and to know which one have highest role.
Rashid Mahmood
Surge Laboratories Private Limited, Pakistan
Title: Hot melt extrusion an emerging drug delivery technology

Biography:
Rashid Mahmood has Master Degree in Analytical Chemistry and MS in Total Quality Management. He has 13 years of experience of Pharmaceutical Quality Ope rations and has attended many international conferences as a keynote speaker and presented various talks in USA & China on Cleaning Validation, cGMP Guidelines, Quality Risk Management etc. Currently he is working as a Senior Executive Manager Quality Operation for Surge Labs.(Manufacturer of Microencapsulated APIs, Liquid & Dry Pow der Parentrals) which is the best export oriented company in Pakistan.
Abstract:
Over the last three decades hot-melt extrusion (HME) has emerged as an influential processing technology in developing molecular dispersions of active pharmaceutical ingredients (APIs) into polymers matrices and has already been demonstrated to provide time controlled, modified, extended and targeted drug delivery resulting in improved bioavailability. HME has now provided opportunity for use of materials in order to mask the bitter taste of active substances. Since industrial application of the extrusion process back in the 1930’s HME has received consider able attention from both the pharmaceutical industry and academia in a range of applications for pharmaceutical dosage forms, such as tablets, capsules, films and implants for drug delivery through oral, transdermal and transmucosal routes. This makes HME an excellent alternative to other conventionally available techniques such as roll spinning and spray drying. In addition to being a proven manufacturing process, HME meets the goal of the US Food and Drug Administration's (FDA) process analytical technology (PAT) scheme for designing, analyzing as well as controlling the manufacturing process through quality control measurements during active extrusion process. The use of hot-melt extrusion (HME) within the pharmaceutical industry is steadily increasing, due to its proven ability to efficiently manufacture novel products. HME involves the application of heat, pressure and agitation through an extrusion channel to mix materials together and subsequently forcing them out through a die. Twin-screw extruders are most popular in solid dosage form development as it imparts both dispersive and distributive mixing. It blends materials while also imparting high shear to break-up particles and disperse them. HME extrusion has been shown to molecularly disperse poorly soluble drugs in a polymer carrier, increasing dissolution rates and bioavailability.
Abdullah Shamim
Khulna University, Bangladesh
Title: Genetic polymorphisms of CYP3A4*1B of cervical cancer patients in Bangladeshi population, Bangladesh

Biography:
To be updated...
Abstract:
Background: Cervical cancer incidence rate in Bangladesh is 15.9 and age-standardized incidence rate is 19.2 (rates per 1,00,000 women per year). Polymorphisms of different genes have been affirmed to be associated with cervical cancer. Our prime purpose is to observe whether CYP3A4*1B polymorphisms are related with increasing cervical cancer risk in Bangladeshi population. The compilation of precise clinical information allows the clarity of different clinical phenotypes, which play a vital role in genetic studies of cervical cancer.
Methods: The study was a case-control study carried out between patients and volunteers matched by age, sex, height, weight and smoking status. Daly’s chemical method was used to isolate genomic DNA from venous blood. The demographic variables of cases and controls were put side by side using chi-square tests and Student’s tests.
Results: Odds ratio and 95 % confidence interval were assigned to estimate the risk of cervical cancer. CYP3A4*1B polymorphisms and cervical cancer risk do not show any considerable relationship. The polymorphic frequencies of CYP3A4*1B allele (normal homozygote, heterozygote and mutant homozygote) in cervical cancer were 60%, 40% and 0% respectively; frequencies in control were 66.67%, 33.33% and 0% respectively (p<0.05). Finally, we conclude that CYP3A4*1B polymorphisms is not associated in susceptibility to developing cervical cancer, at least in Bangladeshi population.
Conclusions: In brief, the consequences of our study demonstrated that CYP3A4*1B polymorphism is not allied in susceptibility to develop cervical cancer, at least in Bangladeshi women.
Tesfaye Zerihun
Addis Ababa University, Ethiopia
Title: Phytochemical screening of the exudate of Aloe otallensis and its effect on Leishmania donovani Parasite

Biography:
To be updated...
Abstract:
Objective: To evaluate antileishmanial activity of methanolic extract of Aloe otallensis (A. otallensis) on the promastigote stage of Leishmania donovani (L. donovani) as compared to standard drugs and to screen its phytochemical constituents.
Methods: Phytochemical screening was done by using the method mentioned by Evans and Trease on methanolic extract of the exudates of Aloe otallensis leaves. The extract was also evaluated for in vitro antileishmanial activity against L. donavani which is found from the Parasitology Unit of Black Lion Hospital. The result was compared to standard drugs of sodium stibogluconate, milfostin and paramomycin.
Results: The extract has a good antileishmanial activity with an IC50 of 0.123 0 μg/mL on L. donovani (AM 563). The experimental data showed that relatively it had better activity than paramomycin and milfostin but less activity than sodium stibogluconate. The data analyses were done by GraphPad Prism version 5 software after it was read by ELISA reader at the wave length of 650 nm. The phytochemical screening of the exudates of A. otallensis showed the presence of phenol, alkaloid and saponin.
Conclusions: The methanol extract of the exudates of A.otallensis has a good anti- leishmaniasis activity and this may be attributed to phenol, alkaloid and saponin present in the plant. But it needs further analysis for the conformation of which constituent presents in high concentration to know which one has the strongest effect.
Akbar Esmaeili
Islamic Azad University, Iran
Title: Vancomycin loaded superparamagnetic MnFe2O4 nanoparticles coated with PEGylated chitosan to enhance antibacterial activity

Biography:
To be updated...
Abstract:
Background & Aims: Increasing prevalence of antibiotic-resistant and failed-treatment make more investigations to deal with these problems. Hence, new therapeutic approaches for effective treatment are necessary. Ferrite superparamagnetic nanoparticles have potentially antibacterial activity.
Methods: In this study, we prepared MnFe2O4 superparamagnetic nanoparticles as core by precipitation method and used chitosan cross linked by glutaraldehyde as shell, then modified with PEG to increase stability of particles against RES.
Results: Chitosan coating not only improves the properties of ferrite nanoparticles but also has antibacterial activity. FT-IR confirmed this surface modification; XRD and SEM were developed to demonstrate particle size and characteristics of crystal structure of these nanoparticles. Final particle size was reported approximately 25 nm. Magnetic properties of nanoparticles were evaluated by VSM. Actual drug loading and releasing were examined by (UV-Vis) spectroscopy method.
Conclusions: We employed liquid broth dilution method to assess antibacterial activity of nanoparticles against microorganisms. Significant antibacterial effect against gram negative bacteria was developed.

