Theme: Emerging Drug Delivery Technologies
Pharmaceutica 2018
ConferenceSeries is a renowned organization that organizes highly notable pharmaceutical conferences throughout the globe. After a successful conference of Pharmaceutica 2017, ConferenceSeries Ltd is currently bringing forth "16th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems" (Pharmaceutica 2018) slated on March 19-21, 2018 at Berlin, Germany.
Pharmaceutica 2018 covers various aspects of Pre-Formulation & Formulation Aspects, Pharmacokinetics and Pharmacodynamics in Drugs, Drug Targeting and Design, Routes of Drug Delivery, Nanoparticulate Drug Delivery Systems, Nanotechnology in Drug Delivery, Pharmaceutical Nanotechnology, Smart Drug Delivery Systems, Biomaterials in Drug Delivery, Vaccine Drug Delivery Systems, Medical Devices for Drug Delivery, Peptides and Protein Drug Delivery, Global Drug Delivery Policy, Entrepreneurs Investment Meet
Track-1: Pre-Formulation & Formulation Aspects
Pharmaceutics is the study of relationships between preformulation, pharmaceutical formulation, delivery, disposition and clinical response. The inherent instability nature of a new drug will alter its desired form into undesired form when presented in a suitable dosage form with the excipient/s upon storage. In early days this process was confined only for assessing few characteristics, but today this process is being considered as a formulation strategy and hence tremendous technological advancement has been achieved in this field which enables us to save time and money through planned management system and hence impacts Pharmaceutica 2018 to be a formulation conference. Use of glorious statistical software even based on artificial neural networking are made the task of preformulation and optimization process easier. Role of preformulation studies techniques like freeze drying aspects projects the event Pharmaceutica 2018 to pose as a freeze drying meeting in drug discovery, drug development plays major role in pharmaceutical formulation development and the studies will help in different dosage forms design. With the increasing number of novel and specialized compounds being developed, a "one size fits all" approach to drug formulation and delivery is no longer optimal, necessitating the consideration of formulations unique to each drug. NDDS conference will discuss on Early Approaches, Present Scenario and Future Prospects of Preformulation events. There are more than 1400 sustained or controlled release drugs have been approved all over the world. Pharmaceutical conferences discuss the state-of-art technology being applied and involve advances in formulation studies.
Revenues within the global generics market reached an estimated value of $265 b in 2012, showing a growth of 9.3% throughout the year. The contribution of generics is approximately 20% of overall international pharmaceutical market. The utilization of generic in terms of volume is higher in the US and lower in Japan, 89% and 24% respectively.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
11th World Drug Delivery Summit, Baltimore, Maryland, USA October 16-18, 2017:20th International Conference on Nanotechnology and Nanomedicine, Prague, Czechia, July 9 - 10, 2018 : 20th International Conference on Antibiotics and Biopharmaceutics, Lisbon, Portugal, April 16 - 17, 2018 : Uncovering Complexity in Drug Metabolism in the Cell and the Clinic, Holderness School 33 Chapel Lane Holderness, NH, US, July 8 - 13, 2018 : International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, Boston USA, April 09-11, 2018:18th World Congress of Basic and Clinical Pharmacology (WCP2018), Kyoto (JP) ,01 - 06 July 2018.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track-2: Drug Targeting and Design
The most fundamental goal in drug design is to predict whether a given molecule will bind to a target and if so how strongly. Molecular mechanics or molecular dynamics are most often used to predict the conformation of the small molecule and to model conformational changes in the biological target that may occur when the small molecule binds to it. The therapeutic response of a drug depends upon the interaction of drug molecules with cell on cell membrane related biological events at receptor sites in concentration dependent manner.
Selective and effective localization of the pharmacologically-active moiety at preidentified target(s) in therapeutic concentration, while restricting its access to non-target(s) normal cellular linings, thus minimizing toxic effects and maximizing the therapeutic index accounts from effective and efficient drug delivery.
Molecular mechanics methods may also be used to provide semi-quantitative prediction of the binding affinity. Also, knowledge-based scoring function may be used to provide binding affinity estimates. These methods use linear regression, machine learning, neural nets or other statistical techniques to derive predictive binding affinity equations by fitting experimental affinities to computationally derived interaction energies between the small molecule and the target.
Ideally, the computational method will be able to predict affinity before a compound is synthesized and hence in theory only one compound needs to be synthesized, saving enormous time and cost. The reality is that present computational methods are imperfect and provide, at best, only qualitatively accurate estimates of affinity. In practice it still takes several iterations of design, synthesis, and testing before an optimal drug is discovered. Computational methods have accelerated discovery by reducing the number of iterations required and have often provided novel structures.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
9th Congress on Drug Design & Drug Formulation, Seoul, South Korea, October 19-20, 2017:19thInternational Conference on Drug Discovery and Design ,Barcelona, Spain, October 30 - 31, 2017 : International Conference On Nanomedicine And Nano biotechnology – ICONAN 2017, Barcelona, September 25-27,2017: International Conference Pharmaceutical & Novel Drug Delivery Systems Double Tree by Hilton Philadelphia Airport, Philadelphia, USA, 07-09 Aug 2017: INTERNATIONAL CONFERENCE ON NANOMEDICINE DRUG DELIVERY, AND TISSUE ENGINEERING (NDDTE'18), BUDAPEST, HUNGARY APRIL 10-12, 2018: Symposium on Emulsion Polymerization and Functional Polymeric Microspheres (ASEPFPM 6), Fukui (JP), 07 - 10 March 2018 :
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track-3: Pharmaceutical Nanotechnology
Size reduction is a fundamental unit operation having important applications in pharmacy. It helps in improving solubility and bioavailability, reducing toxicity, enhancing release and providing better formulation opportunities for drugs. In most of the cases, size reduction is limited to micron size range, for example, various pharmaceutical dosage forms like powder, emulsion, suspension etc. Drugs in the nanometer size range enhance performance in a variety of dosage forms. Major advantages of nanosizing include (i) increased surface area, (ii) enhanced solubility, (iii) increased rate of dissolution, (iv) increased oral bioavailability, (v) more rapid onset of therapeutic action, (vi) less amount of dose required, (vii) decreased fed/fasted variability, and (viii) decreased patient-to-patient variability.
Pharmaceutical nanotechnology has provided more fine-tuned diagnosis and focused treatment of disease at a molecular level. Pharmaceutical nanotechnology is most innovative and highly specialized field, which will revolutionize the pharmaceutical industry in near future. Pharmaceutical nanotechnology presents revolutionary opportunities to fight against many diseases. It helps in detecting the antigen associated with diseases such as cancer, diabetes mellitus, neurodegenerative diseases, as well as detecting the microorganisms and viruses associated with infections. It is expected that in next 10 years market will be flooded with nanotechnology devised medicine.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
12th Congress on Pharmaceutical Sciences and Innovations in Pharma Industry , London, UK, February 26- 27, 2018 : 19th International Conference on Nano medicines and Biomedical Applications, Istanbul, Turkey October 26 - 27, 2017:249th International Conference on Nano science, Nanotechnology and Advanced Materials (IC2NM)Sabah, Sabah, Malaysia, October 10-11,2017: 20th International Conference on Pharmaceutics and Novel Drug Delivery Systems, London, United Kingdom, April 24 - 25, 2018:14th Symposium on Drug Delivery Systems Conference, Lahaina, Maui, Hawaii, December 14-18,2017: 12th Edition of International Conference on Nano Pharmaceutics and Advanced Drug Delivery, Dublin, Ireland, 16-Aug-18.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track -4: Green and Sustainable Pharma:
Green pharmacy is the design of pharmaceutical products and processes that eliminate or reduce significantly the use and generation of hazardous substances and the prevention/reduction of environmental/safety and health impacts at the source. A new pharmaceutical can be green in terms of the quality and quantity of waste generated during its synthesis. Green chemistry was defined by US academics Paul Anastas and John Warner in 1998, who identified 12 principles, including toxicity reduction, biodegradability and energy efficiency, to assess environmental impact. In part, green chemistry is simply an extension of the broader environmental agenda to reduce resource usage and pollution.
Sustainable pharmacy is a totally new issue and approach. It addresses environmental, economical and social aspects of pharmacy. In the present stage the focus will be on environmental issues along the whole lifecycle of a pharmaceutical entity. That is dealing with resources and energy input but also with waste issues for example during the synthesis and production of an Active pharmaceutical Ingredient. Furthermore, it would also look on the compounds themselves and will aim to improve the degradability of the compounds after their use in the environment to reduce the environmental risk caused by pharmaceuticals in the environment. Another issue is the people using pharmaceuticals such as pharmacists, medical doctors and patients. How can they contribute to more efficient use of pharmaceuticals with less environmental burden and less risk for drinking water.
The focus here is on the role of patients, doctors and pharmacists in contributing to green and sustainable pharmacy by proper use of the pharmaceuticals. An environmental classification system for pharmaceuticals as a valuable piece of information for doctors, pharmacists and patients is presented. We also take a look into the future: how will drug consumption develop?
Since green and sustainable pharmacy is still in its infancy, it attempts to identify lack of knowledge and stimulate more activities on the way to sustainable pharmacy rather than to resolve on-going discussions.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
17th International Conference on Nanomedicine and Nanotechnology, Melbourne, Australia, Nov 23-24,2017:20th International Conference on Industrial Pharmacy and Pharmacy Practices, Kuala Lumpur, Malaysia, February 12 - 13, 2018 :Crossing Biological Barriers – Advances in Nano carrier Design for Targeted Drug Delivery, Dresden, Germany, November 9 - 11, 2015 : International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, Boston USA, April 09-11, 2018: CONTROLLED RELEASE DELIVERY ,LONDON, UNITED KINGDOM, 21-22 MARCH 2018.
Related societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track-5: Nanoparticulate Drug Delivery Systems
Nanoparticles (NPs) occur naturally and have been in existence for thousands of years as products of combustion and cooking of food. Nanomaterials differ significantly from other materials due to the following two major principal factors: the increased surface area and quantum effects. These factors can enhance properties such as reactivity, strength, electrical characteristics, and in vivo behaviour. As the particle size decreases, a greater proportion of atoms are found at the surface compared to inside. An NP has a much greater surface area per unit mass compared with larger particles, leading to greater reactivity. In tandem with surface area effects, quantum effects can begin to dominate the properties of matter as size is reduced to the nanoscale. These can affect the optical, electrical, and magnetic behaviour of materials. Their in vivo behaviour can be from increased absorption to high toxicity of nanomaterials. New drug carrier systems are one can name soluble polymers, microparticles made of insoluble (or) biodegradable natural and synthetic polymers, microcapsules, cells, cell ghosts, lipoproteins, liposomes and micelles. Pharmaceutica 2018 evolves to be a drug disintegration conference, emulsion conference, capsule conference, and solubility conference. Pharmaceutical conferences will cover industry case studies, regulatory updates, latest therapies and technology innovations and much more.
Key players in the market include Amgen, Inc., AstraZeneca plc, Eli Lilly & Co., Ipsen S.A., Merck & Co., Novartis AG, Novo Nordisk A/S, Roche Holdings AG, Sanofi, Takeda Pharmaceutical Company Limited, and Teva Pharmaceutical Industries Limited. Leading API manufacturers include Bachem Holding AG, PolyPeptide Group, and Peptisyntha Inc. at the pharmaceutical companies’ conference.
The global market for blood-brain barrier (BBB) technology for therapeutics reached $21.8 million in 2013. This market is expected to grow from $38.7 million in 2014 to $471.5 million in 2019, a compound annual growth rate (CAGR) of 64.9% from 2014 through 2019.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
International Conference on Nano Medicine and Nanoparticles, Las Vegas, USA, April 18-19,2018 :20th International Conference on Nanotechnology and Nanomedicine, Prague, Czechia, July 9 - 10, 2018 : 20th International Conference on Antibiotics and Biopharmaceutics, Lisbon, Portugal, April 16 - 17, 2018 :20th International Conference on Pharmaceutics and Novel Drug Delivery Systems, London, United Kingdom, April 24 - 25, 2018:Lyophilization USA Iselin, NJ, US, November 16-17.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track- 6: Drug Delivery Technologies:
Pharmaceutical drug delivery technologies enhance drug absorption, efficacy, and patient experience. Taste maskers increase the commercial viability of your pharmaceutical products by neutralizing the strong, bitter tastes of certain oral medical formulations. Bioavailability of medications within the system can be achieved by increasing the dissolution rate with specialized drug delivery enhancement products. Enhancing the drug delivery technology of final pharmaceutical formulation can increase its commercial success. The main routes of drug delivery are oral, injection/infusion, and transdermal. Drug-eluting stents and other implantable drug delivery devices are presented, as well as externally applied devices. When combined with appropriate targeting moieties, drug-coated nanoparticles, drug-encapsulating liposomes and nanotubes, and tree-like dendrimers enable organ and tissue targeting.
The use of nanotechnology in medicine and more specifically drug delivery is set to spread rapidly. Currently many substances are under investigation for drug delivery and more specifically for cancer therapy. Interestingly pharmaceutical sciences are using nanoparticles to reduce toxicity and side effects of drugs and up to recently did not realize that carrier systems themselves may impose risks to the patient. The kind of hazards that are introduced by using nanoparticles for drug delivery are beyond that posed by conventional hazards imposed by chemicals in classical delivery matrices
These biotechnology derived drugs were formerly administered by injection alone, but today, solutions for inhaled, transdermal, and even oral delivery are available or under investigation for most established products. Nucleic acid delivery technologies , since unprotected or untargeted delivery of gene therapies or RNAi is inconceivable. We then move on to developments in transdermal delivery technology, which includes active systems where delivery is driven by microneedles or energy applied via ultrasound or lasers.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
14th International Conference on Nanomedicine and Pharmaceutical Nanotechnology Amsterdam, Netherlands, April 09-11, 2018: Uncovering Complexity in Drug Metabolism in the Cell and the Clinic, Holderness School 33 Chapel Lane Holderness, NH, US, July 8 - 13, 2018: International Conference Pharmaceutical & Novel Drug Delivery Systems Double Tree by Hilton Philadelphia Airport, Philadelphia, USA, 07-09 Aug 2017:20th International Conference on Pharmaceutics and Novel Drug Delivery Systems, London, United Kingdom, April 24 - 25, 2018.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA
Track -7:Nanomedicine and Nanotechnology:
Nanotechnology involves the design of structures measuring 1 to 100 nm in diameter. Nanomaterials are being developed as drug-delivery vehicles, contrast agents, and diagnostic devices, and some are now being studied in clinical trials.
The extraordinary developments of nanotechnology and nanomedicine during the past decade have significantly pushed the frontiers of materials science and the inventive spirit of industrial and clinical users alike. The ever improving ability to design, improve and commercialize new materials is clearly a feature that we just started to observe in recent years. Novel nanotechnology is a much needed additional avenue at this time, especially when open access can result from the publication process. The industrialization of nanoscale titanium dioxide for a broad range of applications (most of which we are quite familiar with) is only one such example showing how nanomaterials have become a central component in the manufacturing process during the past decade.
The science of nanotechnology is therefore the ability to manipulate these tiny particles. Nanotechnology is increasingly employed to explore the unseen avenues of medical sciences. There are many types of nanoparticles that are used in medicine and other fields, gold particles, Dendrimers, Perfluorocarbon, Nanotube, Iron oxide and FeCo are just few types that are used in this field and will lead to personalized medicine and early target therapy .
The ability to assemble nanoparticles into functional structures is an important challenge that needs to be addressed for the generation of nanoparticle-based devices.
Sol-gel method represents a facile yet powerful strategy for the self-assembly of metal oxides, chalcogenides, and metal-semiconductor hybrid nanoparticle systems into three-dimensionally connected porous nanostructures. In this highlight, the application of later strategy for the assembly of chalcogenide semiconductor and noble metal nanoparticles and their intriguing physical properties is reviewed in the context of future application in catalysis, sensing, and separation technologies.
By interacting with biological molecules, therefore at nanoscale, nanotechnology opens up a vast field of research and application. Interactions between artificial molecular assemblies or nano devices and biomolecules can be understood both in the extracellular medium and inside the human cells. Operating at nanoscale allows to exploit physical properties different from those observed at microscale such as the volume/surface ratio.
A second area exhibiting a strong development is “nanodrugs” where nanoparticles are designed for targeted drug delivery. The use of such carriers improves the drug bio distribution, targeting active molecules to diseased tissues while protecting healthy tissue. A third area of application is regenerative medicine where nanotechnology allows developing biocompatible materials which support growth of cells used in cell therapy.
The application of nanotechnology to medicine raises new issues because of new uses they allow, for instance.
France is a country where the medical development of nanotechnology is significant, like Germany, the United Kingdom or Spain, as regards the European Union.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
2nd International Conference on Nanomedicine and Drug Delivery,Tokyo, Japan, May 14-16, 2018: 249th International Conference on Nano science, Nanotechnology and Advanced Materials (IC2NM)Sabah, Malaysia, October 10-11,2017: Crossing Biological Barriers – Advances in Nano carrier Design for Targeted Drug Delivery, Dresden, Germany, November 9 - 11, 2015 : 19th International Conference on Drug Design and Pharmacokinetics, London, United Kingdom, July 24 - 25, 2017.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track-8: Smart Drug Delivery Systems
Smart drug delivery is a method of delivering medication to a patient in a manner that increases the concentration of the medication in some parts of the body relative to others. This means of delivery is largely founded on nanomedicine, which plans to employ nanoparticle-mediated drug delivery in order to combat the downfalls of conventional drug delivery. These nanoparticles would be loaded with drugs and targeted to specific parts of the body where there is solely diseased tissue, thereby avoiding interaction with healthy tissue. The goal of a targeted drug delivery system is to prolong, localize, target and have a protected drug interaction with the diseased tissue. The conventional drug delivery system is the absorption of the drug across a biological membrane, whereas the targeted release system releases the drug in a dosage form. The advantages to the targeted release system is the reduction in the frequency of the dosages taken by the patient, having a more uniform effect of the drug, reduction of drug side-effects, and reduced fluctuation in circulating drug levels. The disadvantage of the system is high cost, which makes productivity more difficult and the reduced ability to adjust the dosages.
Targeted drug delivery systems have been developed to optimize regenerative techniques. The system is based on a method that delivers a certain amount of a therapeutic agent for a prolonged period of time to a targeted diseased area within the body. This helps maintain the required plasma and tissue drug levels in the body, thereby preventing any damage to the healthy tissue via the drug. The drug delivery system is highly integrated and requires various disciplines, such as chemists, biologists, and engineers, to join forces to optimize this system.
The global revenue for advanced drug delivery systems is estimated to be $151.3 billion in 2013. In 2018, revenues are estimated to reach nearly $173.8 billion, demonstrating a compound annual growth rate (CAGR) of 2.8%.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
15th International Conference on Pharmaceutical Formulations Rome, Italy, July 26-27 2018: Global Drug Delivery & Formulation Summit (DDF), Berlin, Germany, March 12-14:18th World Congress of Basic and Clinical Pharmacology (WCP2018), Kyoto (JP), 01 - 06 July 2018: Symposium on Emulsion Polymerization and Functional Polymeric Microspheres (ASEPFPM 6), Fukui (JP) ,07 - 10 March 2018 : 12th Edition of International Conference on Nano Pharmaceutics and Advanced Drug Delivery, Dublin, Ireland, 16-Aug-18: CONTROLLED RELEASE DELIVERY ,LONDON, UNITED KINGDOM, 21-22 MARCH 2018: International Conference and Exhibition on Nanomedicine and Drug Delivery, Tokyo, Japan ,May 14-16, 2018.
Track-9: Biomaterials in Drug Delivery
Biomaterials are any substance that has been engineered to interact with biological systems for a medical purpose - either a therapeutic (treat, augment, repair or replace a tissue function of the body) or a diagnostic one. Biomaterials can be derived either from nature or synthesized in the laboratory using a variety of chemical approaches utilizing metallic components, polymers, ceramics or composite materials. They are often used and/or adapted for a medical application, and thus comprise whole or part of a living structure or biomedical device which performs, augments, or replaces a natural function. Such functions may be benign, like being used for a heart valve, or may be bioactive with a more interactive functionality such as hydroxy-apatite coated hip implants. Biomaterials are also used every day in dental applications, surgery, and drug delivery. For example, a construct with impregnated pharmaceutical products can be placed into the body, which permits the prolonged release of a drug over an extended period of time. A biomaterial may also be an autograft, allograft or xenograft used as a transplant material. Pharmaceutica 2018 have penetrated into the biomaterials realm and hence a biomaterials conference.
Global revenue for vaccine technologies was nearly $31.8 billion in 2011. This market is expected to increase from $33.6 billion in 2012 to $43.4 billion in 2017 at a compound annual growth rate (CAGR) of 5.3%.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
15th Pharma Congress , Melbourne, Australia, July 16-18, 2018: International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, Boston USA, April 09-11, 2018: 3RD INTERNATIONAL CONFERENCE ON NANOMEDICINE DRUG DELIVERY, AND TISSUE ENGINEERING (NDDTE'18), BUDAPEST, HUNGARY APRIL 10 - 12, 2018:14th Symposium on Drug Delivery Systems Conference, Lahaina, Maui, Hawaii, December 14-18,2017: Inhalation & Respiratory Drug Products Summit, Berlin, Germany, September 28-29: Lyophilization USA Iselin, NJ, US, November 16-17: Drug Delivery to the Lungs 2017 (DDL2017),Edinburgh, UK, December 6-8:9th Global Drug Delivery & Formulation Summit (DDF) , Berlin, Germany, March 12-14.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track-10: Vaccine Drug Delivery Systems
Vaccine is a material that induces an immunologically mediated resistance to a disease but not necessarily an infection. Vaccines are generally composed of killed or attenuated organisms or subunits of organisms or DNA encoding antigenic proteins of pathogens. Sub-unit vaccines though exceptionally selective and specific in reacting with antibodies often fail to show such reactions in circumstances such as shifts in epitopic identification center of antibody and are poorly immunogenic. Delivery of antigens from oil-based adjuvants such as Freunds adjuvant lead to a reduction in the number of doses of vaccine to be administered but due to toxicity concerns like inductions of granulomas at the injection site, such adjuvants are not widely used. FDA approved adjuvants for human uses are aluminium hydroxide and aluminium phosphate in the form of alum. Hence, search for safer and potent adjuvants resulted in the formulation of antigen into delivery systems that administer antigen in particulate form rather than solution form.
Other reasons driving the development of vaccines as controlled drug delivery systems are as follows:
- Immunization failure with conventional immunization regimen involving prime doses and booster doses, as patients neglect the latter. Vaccines delivery systems on the other hand:
- Allow for the incorporation of doses of antigens so that booster doses are no longer necessary as antigens are released slowly in a controlled manner.
- Control the spatial and temporal presentation of antigens to the immune system there by promoting their targeting straight to the immune cells.
The global market for pharmaceutical and biopharmaceutical contract manufacturing, research and packaging was $219.9 billion in 2012. This market is estimated to reach $242.2 billion in 2013 and $374.8 billion by 2018, a five-year compound annual growth rate (CAGR) of 9.1%.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
17thInternational Conference on Nanomedicine and Nanotechnology, Melbourne, Australia, Nov 23-24,2017: Uncovering Complexity in Drug Metabolism in the Cell and the Clinic, Holderness School 33 Chapel Lane Holderness, NH, US, July 8 - 13, 2018 : International Conference Pharmaceutical & Novel Drug Delivery Systems Double Tree by Hilton Philadelphia Airport, Philadelphia, USA, 07-09 Aug 2017: 20th International Conference on Pharmaceutics and Novel Drug Delivery Systems, London, United Kingdom, April 24 - 25, 2018:
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track-11: Medical Devices for Drug Delivery
A medical device is any instrument, apparatus, appliance, software, material or other article, whether used alone or in combination, including the software intended by its manufacturer to be used specifically for diagnostic and/or therapeutic purposes and necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of:
· Diagnosis, prevention, monitoring, treatment or alleviation of disease;
· Diagnosis, monitoring, treatment, alleviation of or compensation for an injury or handicap;
· Investigation, replacement or modification of the anatomy or of a physiological process;
Medical devices vary according to their intended use and indications. Examples range from simple devices such as tongue depressors, medical thermometers, and disposable gloves to advanced devices such as computers which assist in the conduct of medical testing, implants, and prostheses. The design of medical devices constitutes a major segment of the field of mechanical engineering. Pharmaceutica 2018 is considered to be a medical devices conference.
The drug-device combination market is not fragmented and the key players in this market are Medtronic, Boston Scientific Corp., Edwards Life sciences Corp., Stryker Corp., QLT Inc. etc. The maximum number of new product developments is expected to take place in the bone graft substitutes, advanced wound care products and antimicrobial catheter markets. Our patent analysis indicates that E.U. has filed for the maximum number of patents followed by the U.S.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
12th Congress on Pharmaceutical Sciences and Innovations in Pharma Industry , London, UK, February 26- 27, 2018 : International Conference Pharmaceutical & Novel Drug Delivery Systems Double Tree by Hilton Philadelphia Airport, Philadelphia, USA, 07-09 Aug 2017: 20th International Conference on Pharmaceutics and Novel Drug Delivery Systems, London, United Kingdom, April 24 - 25, 2018.
Track-12: Peptides and Protein Drug Delivery
Peptides and proteins have great potential as therapeutics. Peptides can be designed to target a broad range of molecules, giving them almost limitless possibilities in fields such as oncology, immunology, infectious disease and endocrinology. While the peptide and protein therapeutic market has developed significantly in the past decades, delivery has limited their use. Although oral delivery is preferred, most are currently delivered intravenously or subcutaneously due to degradation and limited absorption in the gastrointestinal tract. Therefore, absorption enhancers, enzyme inhibitors, carrier systems and stability enhancers are being studied to facilitate oral peptide delivery. Additionally, transdermal peptide delivery avoids the issues of the gastrointestinal tract, but also faces absorption limitations. Due to proteases, opsonization and agglutination, free peptides are not systemically stable without modifications.
Currently, the market for peptide and protein drugs is estimated to be greater than US$40 billion/year, or 10% of the pharmaceutical market. This market is growing much faster than that of small molecules, and will make up an even larger proportion of the market in the future. At present there are over 100 approved peptide-based therapeutic drugs on the market, with the majority being smaller than 20 amino acids. Compared with the typical small-molecule drugs that currently make up the majority of the pharmaceutical market, peptides and proteins can be highly selective as they have multiple points of contact with their target. Increased selectivity may also result in decreased side effects and toxicity.
Major drugs driving growth of the overall smart drug delivery market include Angiomax, Copaxone, Forteo, Sandostatin, Velcade, Victoza and Zoladex.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
12th Congress on Pharmaceutical Sciences and Innovations in Pharma Industry , London, UK, February 26- 27, 2018 : Symposium on Emulsion Polymerization and Functional Polymeric Microspheres (ASEPFPM 6), Fukui (JP) ,07 - 10 March 2018 : 12th Edition of International Conference on Nano Pharmaceutics and Advanced Drug Delivery, Dublin, Ireland, 16-Aug-18: CONTROLLED RELEASE DELIVERY ,LONDON, UNITED KINGDOM, 21-22 MARCH 2018: International Conference and Exhibition on Nanomedicine and Drug Delivery, Tokyo, Japan, May 14-16, 2018.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA
TRACK -13 :2D & 3D Printing in Drug Delivery:
There has been increased activity in the field recently regarding the development and research on various printing techniques in fabrication of dosage forms and drug delivery systems. These technologies may offer benefits and flexibility in manufacturing, potentially paving the way for personalized dosing and tailor-made dosage forms.
2D and 3D printing permits fabricating functional structures of metals, polymers, and biomaterials. 3D structures can be printed on a variety of surfaces with characteristic permeability, porosity, hydrophobicity/hydrophilicity and surface energy. This allows controlling the properties of the printed substances. Besides accurate patterning, printing on tailored functionalized substrates and multi-layer printing makes automated high-speed manufacture of complex structures possible. It opens up new avenues for tailoring physicochemical properties of organic substances.
3D-printing (3DP) is the art and science of printing in a new dimension using 3D printers to transform 3D computer aided designs (CAD) into life-changing products. This includes the design of more effective and patient-friendly pharmaceutical products as well as bio-inspired medical devices. It is poised as the next technology revolution for the pharmaceutical and medical-device industries. After decorous implementation scientists in collaboration with CAD designers have produced innovative medical devices ranging from pharmaceutical tablets to surgical transplants of the human face and skull, spinal implants, prosthetics, human organs and other biomaterials. a limitation exists in the availability of 3D printable biomaterials for most applications.
With the FDA approval of the first 3D printed tablet, Spritam®, there is now precedence set for the utilization of 3D printing for the preparation of drug delivery systems.
The high degree of flexibility and control with 3D printing enables the preparation of dosage forms with multiple active pharmaceutical ingredients with complex and tailored release profiles. A unique opportunity for this technology for the preparation of personalized doses to address individual patient needs. This review will highlight the 3D printing technologies being utilized for the fabrication of drug delivery systems, as well as the formulation and processing parameters for consideration.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
12th Congress on Pharmaceutical Sciences and Innovations in Pharma Industry , London, UK, February 26- 27, 2018 :14th Symposium on Drug Delivery Systems Conference, Lahaina, Maui, Hawaii, December 14-18,2017: Inhalation & Respiratory Drug Products Summit, Berlin, Germany, September 28-29: Lyophilization USA Iselin, NJ, US, November 16-17: Drug Delivery to the Lungs 2017 (DDL2017),Edinburgh, UK ,December 6-8: 9th Global Drug Delivery & Formulation Summit (DDF) , Berlin, Germany, March 12-14.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track-14: Pharmacokinetics and Pharmacodynamics in Drugs
Pharmacokinetics is currently defined as the study of the time course of drug absorption, distribution, metabolism, and excretion. Clinical pharmacokinetics is the application of pharmacokinetic principles to the safe and effective therapeutic management of drugs in an individual patient. Primary goals of clinical pharmacokinetics include enhancing efficacy and decreasing toxicity of a patient’s drug therapy. The development of strong correlations between drug concentrations and their pharmacologic responses has enabled clinicians to apply pharmacokinetic principles to actual patient situations.
Pharmacodynamics refers to the relationship between drug concentration at the site of action and the resulting effect, including the time course and intensity of therapeutic and adverse effects. The effect of a drug present at the site of action is determined by that drug’s binding with a receptor. Receptors may be present on neurons in the central nervous system (i.e., opiate receptors) to depress pain sensation, on cardiac muscle to affect the intensity of contraction, or even within bacteria to disrupt maintenance of the bacterial cell wall.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
2ndInternational Conference on Nanomedicine and Drug Delivery ,Tokyo, Japan, May 14-16, 2018: International Conference Pharmaceutical & Novel Drug Delivery Systems Double Tree by Hilton Philadelphia Airport, Philadelphia, USA, 07-09 Aug 2017: 20th International Conference on Pharmaceutics and Novel Drug Delivery Systems, London, United Kingdom, April 24 - 25, 2018.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track-15: Routes of Drug Delivery
A route of administration is the path by which a drug, fluid, poison, or other substance is taken into the body. Routes of administration are generally classified by the location at which the substance is applied. Common examples include oral and intravenous administration. Routes can also be classified based on where the target of action is. Action may be topical (local), enteral (system-wide effect, but delivered through the gastrointestinal tract), or parenteral (systemic action, but delivered by routes other than the GI tract).
Routes of administration are usually classified by application location (or exposition). The route or course the active substance takes from application location to the location where it has its target effect is usually rather a matter of pharmacokinetics (concerning the processes of uptake, distribution, and elimination of drugs). Nevertheless, some routes, especially the transdermal or transmucosal routes are commonly referred to routes of administration. The location of the target effect of active substances is usually rather a matter of pharmacodynamics (concerning e.g. the physiological effects of drugs). Furthermore, there is also a classification of routes of administration that basically distinguishes whether the effect is local (in "topical" administration) or systemic (in "enteral" or "parenteral" administration).
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
11th World Drug Delivery Summit, Baltimore, Maryland, USA October 16-18, 2017:18th World Congress of Basic and Clinical Pharmacology (WCP2018), Kyoto (JP), 01 - 06 July 2018: Symposium on Emulsion Polymerization and Functional Polymeric Microspheres (ASEPFPM 6), Fukui (JP), 07 - 10 March 2018 : 12th Edition of International Conference on Nano Pharmaceutics and Advanced Drug Delivery, Dublin, Ireland, 16-Aug-18: CONTROLLED RELEASE DELIVERY ,LONDON, UNITED KINGDOM, 21-22 MARCH 2018: International Conference and Exhibition on Nanomedicine and Drug Delivery, Tokyo, Japan, May 14-16, 2018.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track-16: Nanotechnology in Drug Delivery
Nanotechnology has finally and firmly entered the realm of drug delivery. Performances of intelligent drug delivery systems are continuously improved with the purpose to maximize therapeutic activity and to minimize undesirable side-effects. The primary goals for research of nano-bio-technologies in drug delivery include:
- More specific drug targeting and delivery,
- Reduction in toxicity while maintaining therapeutic effects,
- Greater safety and biocompatibility, and
- Faster development of new safe medicines
Pharmaceutical conferences offers presentations by researchers from a number of disciplines, from the life sciences to engineering, who will address a range of topics including peptide and protein delivery, gene delivery, cell delivery, vaccines, transdermals, pulmonary delivery, new materials, and other subjects, from varied disciplines while focusing on the central theme of drug delivery.
Key players in the market include Amgen, Inc., AstraZeneca plc, Eli Lilly & Co., Ipsen S.A., Merck & Co., Novartis AG, Novo Nordisk A/S, Roche Holdings AG, Sanofi, Takeda Pharmaceutical Company Limited, and Teva Pharmaceutical Industries Limited. Leading API manufacturers include Bachem Holding AG, PolyPeptide Group, Peptisyntha Inc. and Lonza Inc.
The global market for blood-brain barrier (BBB) technology for therapeutics reached $21.8 million in 2013. This market is expected to grow from $38.7 million in 2014 to $471.5 million in 2019, a compound annual growth rate (CAGR) of 64.9% from 2014 through 2019.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, Boston USA, April 09-11, 2018: INTERNATIONAL CONFERENCE ON NANOMEDICINE DRUG DELIVERY, AND TISSUE ENGINEERING (NDDTE'18), BUDAPEST, HUNGARY APRIL 10 - 12, 2018: 14th Symposium on Drug Delivery Systems Conference, Lahaina, Maui, Hawaii, December 14-18,2017: Inhalation & Respiratory Drug Products Summit, Berlin, Germany, September 28-29: Lyophilization USA Iselin, NJ, US, November 16-17: Drug Delivery to the Lungs 2017 (DDL2017),Edinburgh, UK, December 6-8: 9th Global Drug Delivery & Formulation Summit (DDF) , Berlin, Germany, March 12-14: 9th Congress on Drug Design & Drug Formulation, Seoul, South Korea, October 19-20, 2017 .
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
Track-17: Global Drug Delivery Policy
The global market for Business Development of Drug Delivery Technology in 2010 was $131.6 billion and is expected to rise at a compound annual growth rate (CAGR) of 5% and reach nearly $175.6 billion by 2016. The U.S. constituted approximately 59% of the total drug delivery market in 2010 and was $78 billion. It is forecast to reach nearly $103 billion in 2016 at a CAGR of 4.7%. Europe contributed about 27% of the total drug delivery market in 2010 and was $36 billion and is expected to grow to $49 billion by 2016 at a CAGR of 5.6% for 2013, Drug Delivery Global market reached $150.3 billion, according to BCC research. This was an increase from $142 billion the previous year. Given its predicted annual growth the market represents a considerable business opportunity, which has been reflected in the increasing number of drug delivery specialists.
Consistent quality and competitive costs of product improves Production performance and continuity of supply and Product and technology auditing and due diligence with minimizing Regulatory Issues, quality control, and business development Business opportunities in drug delivery. Pharmaceutical conferences offer unparalleled opportunities to establish new business relationships with companies from 40 countries through pre-scheduled one-on-one meetings with decision makers.
Related Pharmaceutics Conferences | Drug Delivery Conferences | NDDS Conferences | Drug Delivery Symposiums | Drug Delivery Workshops | Formulations Conferences
2nd International Conference on Nanomedicine and DrugDelivery,Tokyo, Japan ,May 14-16, 2018:INTERNATIONAL CONFERENCE ON NANOMEDICINE DRUG DELIVERY, AND TISSUE ENGINEERING (NDDTE'18), BUDAPEST, HUNGARY APRIL 10 - 12, 2018: 14th Symposium on Drug Delivery Systems Conference, Lahaina, Maui, Hawaii, December 14-18,2017: Inhalation & Respiratory Drug Products Summit, Berlin, Germany, September 28-29.
Related Societies:
Controlled Release Society-CRS; Society of Biomaterials; International Society of Drug Delivery Sciences and Technology-APGI; Japan Society of Drug Delivery System; Oral Drug Delivery Focus Group; The Aerosol Society; Parenteral Drug Association-PDA; American Association of Pharmaceutical Scientists-AAPS; International Pharmaceutical Federation-FIP; Inhalation Drug Delivery Association-IDDA.
ConferenceSeries is a renowned organization that organizes highly notable pharmaceutical conferences throughout the globe.
After a successful conference of Pharmaceutica 2017, ConferenceSeries is currently bringing forth "16th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems" (Pharmaceutica 2018) slated on March 19-21, 2018 at Berlin, Germany. The conference invites all the participants across the globe to attend and share their insights and convey recent developments in the field of Pharmaceutics and Novel Drug Delivery Systems.
2018 Highlights:
- 200+ Participation (70% Industry: 30% Academia)
- 9+ Keynote Speakers
- 30+ Plenary Speakers
- 3+ Exhibitors
- 14 Innovative Educational Sessions
- B2B Meetings
Exhibition:
Why exhibit?
- Make sales
- Debut new products
- Profile your brand
- Meet new business partners and suppliers
- Develop key relationships
- Educate healthcare, pharma and biotech institutions and academia
Who you will meet?
Leaders in:
- Drug Delivery
- Formulation
- Drug Development
- Nanotechnology
- QbD
- Delivery Devices
- Bioavailability
- New Products
- Pre-Formulation
- Process R&D
- CMC
- Stability
- Bio manufacturing
- Manufacturing
- Solid State Chemistry
- Analytical Development
- Product Enhancement
Who should sponsor?
- Analytical Services
- Formulation Development
- CMO
- Drug Delivery Technologies
- Full service CRO
- Preformulation testing
- Software
Why you should attend?
- Get to the heart of why formulation and delivery strategies fail. Dissect the challenges before looking for concrete solutions.
- Discover how advances in the sector are impacting both large and small molecule drugs.
- Explore tried and tested routes to improve bioavailability.
- Understand how to develop the right formulation and delivery strategy with a strong scientific, clinical and commercial mind set.
- Discover the latest innovations in drug delivery devices.
- Be inspired by innovative case studies and realise the potential impact on your formulation or delivery processes.
- Engage in the exciting event format, with round tables, panels, showcases, speed networking and multiple conference tracks.
- Share experiences, insights and strategies in interactive peer-to peer round tables.
- Hear more perspectives in one place – from large medium and small organisations from pharma, biotech and academia.
- Discover how scientific formulation advancements are being implemented in practice.
- Get to the heart of why formulation and delivery strategies fail. Dissect the challenges before looking for concrete solutions.
ConferenceSeries is a renowned organization that organizes highly notable pharmaceutical conferences throughout the globe.
After a successful conference of Pharmaceutica 2017, ConferenceSeries Ltd is currently bringing forth "16th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems" (Pharmaceutica 2018) slated on March 19-21, 2018 at Berlin, Germany. The conference invites all the participants across the globe to attend and share their insights and convey recent developments in the field of Pharmaceutics and Novel Drug Delivery Systems.
2018 Highlights:
- 200+ Participation (70% Industry: 30% Academia)
- 9+ Keynote Speakers
- 30+ Plenary Speakers
- 3+ Exhibitors
- 14 Innovative Educational Sessions
- B2B Meetings
Exhibition:
Why exhibit?
- Make sales
- Debut new products
- Profile your brand
- Meet new business partners and suppliers
- Develop key relationships
- Educate healthcare, pharma and biotech institutions and academia
Who you will meet?
Leaders in:
- Drug Delivery
- Formulation
- Drug Development
- Nanotechnology
- QbD
- Delivery Devices
- Bioavailability
- New Products
- Pre-Formulation
- Process R&D
- CMC
- Stability
- Bio manufacturing
- Manufacturing
- Solid State Chemistry
- Analytical Development
- Product Enhancement
Who should sponsor?
- Analytical Services
- Formulation Development
- CMO
- Drug Delivery Technologies
- Full service CRO
- Preformulation testing
- Software
Why you should attend?
- Get to the heart of why formulation and delivery strategies fail. Dissect the challenges before looking for concrete solutions.
- Discover how advances in the sector are impacting both large and small molecule drugs.
- Explore tried and tested routes to improve bioavailability.
- Understand how to develop the right formulation and delivery strategy with a strong scientific, clinical and commercial mind set.
- Discover the latest innovations in drug delivery devices.
- Be inspired by innovative case studies and realise the potential impact on your formulation or delivery processes.
- Engage in the exciting event format, with round tables, panels, showcases, speed networking and multiple conference tracks.
- Share experiences, insights and strategies in interactive peer-to peer round tables.
- Hear more perspectives in one place – from large medium and small organisations from pharma, biotech and academia.
- Discover how scientific formulation advancements are being implemented in practice.
- Get to the heart of why formulation and delivery strategies fail. Dissect the challenges before looking for concrete solutions.
Pharmaceutics & Drug Delivery Market Research:
Pharmaceuticals are one of the world's most profitable industries. During the last 30 years, the industry has spent billions of dollars on research and reaped billions in return. In 2008 alone, the pharmaceutical industry sold $773 billion in products worldwide-a number that has consistently grown for the past 8 years and is projected to increase again by 2.5 to 3.5 percent in 2017.
*Source: IMS Health
The Market report of most of the pharmaceutical companies shows their R&D investments majorly in the following areas, namely:
- Advanced Drug Delivery Systems
- Antibiotics
- Excipients in Pharmaceuticals
- Ophthalmic Therapeutic Drugs
- Skin Disease Treatment
- Vaccine Technologies
- Breakthrough Therapies
Advanced Drug Delivery Systems:
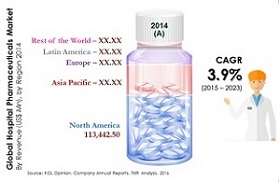
- The global advanced drug delivery market should grow from roughly $178.8 billion in 2015 to nearly $227.3 billion by 2020, with a compound annual growth rate (CAGR) of 4.9%.
- The North American market should grow from nearly $75.7 billion in 2015 to $93.4 billion by 2020, a CAGR of 4.3%.
- The European market should grow from roughly $57.3 billion in 2015 to nearly $72.1 billion by 2020, a CAGR of 4.7%.
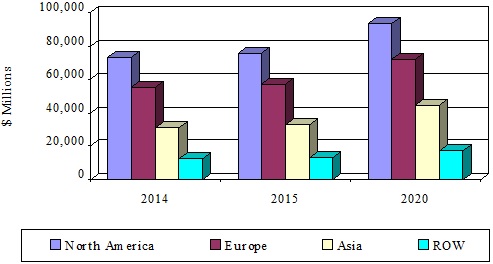
*Source: BCC Research
Antibiotics:
- The global systemic antibiotics market should reach nearly $44.7 billion in 2020 from nearly $40.6 billion in 2015 at a compound annual growth rate (CAGR) of 2.0% from 2015 to 2020.
- The beta-lactams market should reach over $22.0 billion by 2020 from over $20.6 billion in 2015, a CAGR of 1.3% from 2015 to 2020.
- The other antibiotic classes market should reach over $10.6 billion in 2020 from nearly $8.3 billion in 2015, a CAGR of 5.0% from 2015 to 2002.
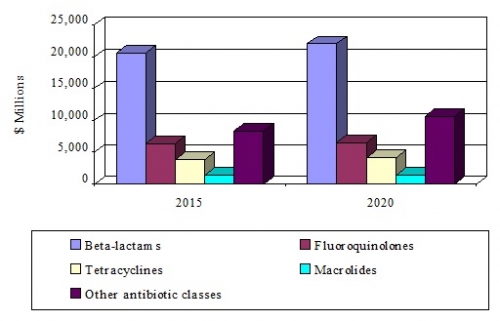
*Source: BCC Research
Excipients in Pharmaceuticals:
- The global excipients market should reach nearly $6.9 billion by 2020 from over $6.2 billion in 2015, a compound annual growth rate (CAGR) of 1.9% from 2015 to 2020.
- The organic excipients market should reach $6.3 billion by 2020 from nearly $5.8 billion in 2015, a CAGR of 1.7% from 2015 to 2020.
- The inorganic excipients market should reach $433.7 million by 2020 from $351.9 million in 2015, a CAGR of 4.3% from 2015 to 2020.
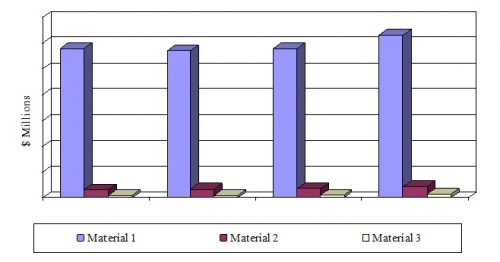
*Source: BCC Research
Ophthalmic Therapeutic Drugs:
- The global ophthalmic therapeutic drug market was valued at $12.3 billion in 2014. This market is expected to reach $19 billion by 2019, with a compound annual growth rate (CAGR) of 9.1% from 2014 to 2019.
- The global age-related macular degeneration (AMD) market is expected to grow to nearly $7.9 billion by 2019 from nearly $5.4 billion in 2014, a CAGR of 7.8% from 2014 to 2019.
- The global glaucoma therapeutic market generated revenue of $3.8 billion in 2014, and by 2019 this segment is expected to generate $6.4 billion, with a CAGR of 11.1% from 2014 to 2019.
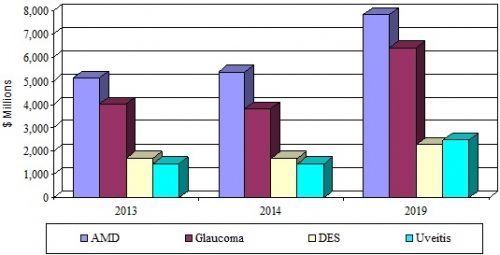
*Source: BCC Research
Skin Disease Treatment:
- The global market for skin disease treatment technologies reached $16.4 billion in 2014 and $17.1 billion in 2015. The market should reach $20.4 billion in 2020, demonstrating a compound annual growth rate (CAGR) of 3.6% from 2015 to 2020.
- The U.S market for skin disease treatment dominates the global market throughout the period, and totaled $7.3 billion in 2014. The market should reach $7.5 billion in 2015 and $8.6 billion in 2020 at a CAGR of 2.6% from 2015 to 2020.
- BRIC (Brazil, Russia, India, and China) is the fastest growing region of the global dermatology market with a CAGR of 6% from 2015 to 2020. The market totaled $3.5 billion in 2015 and should reach more than $4.6 billion by 2020.
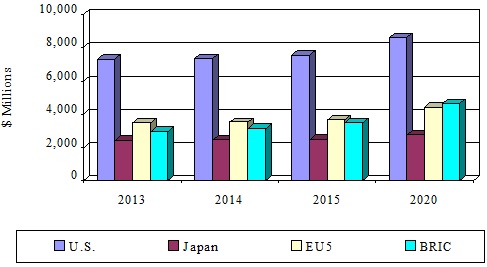
*Source: BCC Research
Vaccine Technologies:
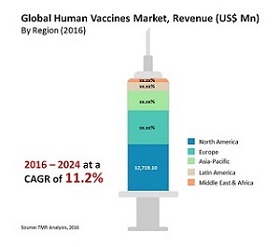
- The global market for vaccine technologies reached $33.3 billion in 2016 and should reach $45.2 billion by 2021, growing at a compound annual growth rate (CAGR) of 6.3% from 2016 to 2021.
- Human vaccines as a segment of this market reached $27.2 billion in 2016 and should reach nearly $37.5 billion by 2021, growing at a CAGR of 6.6% from 2016 to 2021.
- Animal vaccines as a segment of this market reached nearly $6.1 billion in 2016 and should reach $7.7 billion by 2021, growing at a CAGR of 4.9% from 2016 to 2021.
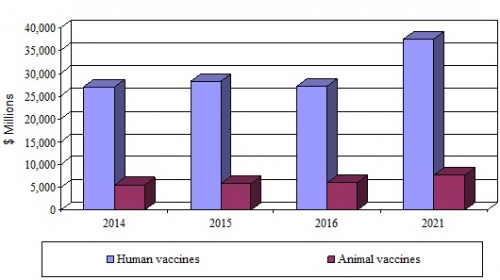
*Source: BCC Research
Breakthrough Therapies:
- The global market for breakthrough therapy designation (BTD) drugs should reach $99.2 billion by 2022 from $48.8 billion in 2017 at a compound annual growth rate (CAGR) of 15.2%, from 2017 to 2022.
- The cancer therapy segment of the breakthrough therapy designation drugs industry is the largest market. The market is expected to grow from $19.6 billion in 2017 to $58.6 billion in 2022 at a CAGR of 24.5% for the period 2017-2022.
- The CNS and neurology therapy segment of the breakthrough therapy designation drugs industry is expected to grow from $956 million in 2017 to $8.4 billion in 2022 at a CAGR of 54.3% for the period 2017-2022.
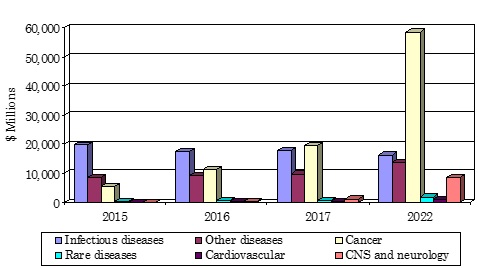
*Source: BCC Research
2017 Pharmaceuticals Research Review:
Pharmaceuticals represented a US$300 bn-a-year market globally as of 2015, the World Health Organization states. The global pharmaceutical market is expected to surpass US$400 bn by 2018, with the ten largest pharmaceutical companies collectively commanding about a third of the market. Companies in the pharmaceutical industry are characterized by their sizeable expenditure on R&D and marketing initiatives in a bid to rake in more revenue. The development of biopharmaceuticals represents a milestone for the industry and personalized therapies carry immense promise in the near future.
Highlights:
- The global market for cancer vaccines totaled $4.5 billion in 2013 and reached nearly $4.0 billion in 2014. This market is expected to reach $4.3 billion by 2019, registering a compound annual growth rate (CAGR) of 1.3% for the period 2014-2019.
- The global market for “silent” cancers was valued at $8.5 billion in 2013 and $9 billion in 2014. This market is expected to reach almost $13.6 billion by 2019, with a CAGR of 8.5% from 2014 to 2019.
- The global market for incretin-based therapeutics was valued at nearly $11.8 billion in 2013 and $12.7 billion in 2014. This market is expected to reach $22.8 billion by 2019, with a CAGR of 12.4% from 2014 to 2019.
Cancer Vaccines: Technologies and Global Markets:
The global human vaccines market was valued at US$28.3 bn in 2015 and is estimated to reach US$72.5 bn by 2024, registering an 11.2% CAGR during the forecast period. Extensive promotion and marketing strategies and favourable government support are likely to boost market growth.
The global market for cancer vaccines was valued at nearly $4.2 billion and $4.0 billion in 2014 and 2015, respectively. This market is expected to decline from nearly $3.5 billion in 2016 to $3.4 billion in 2021 at a compound annual growth rate (CAGR) of -0.4% for 2016-2021.
Drug Delivery Companies:
The global drug delivery technology market is projected to reach USD 1,669.40 Billion by 2021 from USD 1,179.20 Billion in 2016, at a CAGR of 7.2% during the forecast period. This market is segmented based on route of administration, facility of use, and region.
The drug delivery technology is highly competitive market, comprising of various players. Prominent players in the drug delivery technology market include Johnson & Johnson, Inc. (U.S.), F. Hoffman-La Roche (Switzerland), Merck & Co., Inc. (U.S.), Bayer AG (Germany), Pfizer, Inc. (U.S.), Novartis AG (Switzerland), 3M Company (U.S.), Becton, Dickinson and Company (U.S.), GlaxoSmithKline plc, (U.K.), Sanofi (France), and Antares Pharma, Inc. (U.S.).
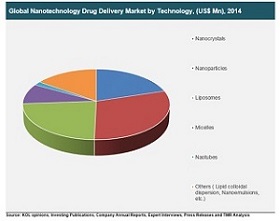
Nanotechnology Drug Delivery Market:
The global nanotechnology drug delivery market was valued at US$ 41,062.5 Mn in 2014 and is projected to reach US$ 118,527.2 Mn by 2023, expanding at a CAGR of 12.5% from 2015 to 2023.
Key players having presence in the global nanotechnology drug delivery market are AbbVie, Inc., Amgen, Inc., Celgene Corporation, Johnson & Johnson, Merck & Co., Inc., and Novartis International AG, among others.
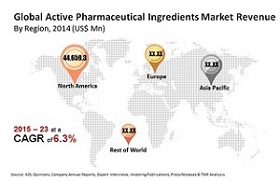
Active Pharmaceutical Ingredient Market:
Transparency Market research states the opportunity in this market will be worth US$219.60 bn by 2023 from US$134.7 bn in 2015. Between the forecast period of 2015 and 2023, the overall market is expected to expand at a CAGR of 6.3%.
Some of the leading companies operating in the global active pharmaceutical ingredient market are Zhejiang Medicine Co., Ltd., Teva Pharmaceutical Industries Ltd., Zhejiang NHU Co., Ltd , North China Pharmaceutical Group Corp. (NCPC), Dr.Reddy’s Laboratories Limited, Northeast Pharmaceutical Group Co., Ltd., Aurobindo Pharma Limited, Sandoz (Novartis AG), Zhejiang Hisun Pharmaceutical Co., Ltd. and Zhejiang Huahai Pharmaceuticals Co., Ltd. amongst others. Currently, these players are focusing on partnering with other companies to acquire a new line of products to add value to their portfolio. Furthermore, companies are also anticipated to focus on expanding their capacities to cater to the vast unmet medical needs of the world.
Target Audience:
- Drug Delivery Technology Manufacturers
- Public and Private Physicians
- Healthcare Institutions (Medical Data Centers)
- Research & Clinical Laboratories
- Distributors and Suppliers of Drug Delivery Technologies
- Health Insurance Payers
- Market Research and Consulting Firms
Conference Highlights
- Drug Targeting and Design
- Drug Delivery Technologies
- Nanoparticulate Drug Delivery Systems
- Pharmaceutical Nanotechnology
- Nanomedicine and Nanotechnology
- Smart Drug Delivery Systems
- Biomaterials in Drug Delivery
- Vaccine Drug Delivery Systems
- Medical Devices for Drug Delivery
- Peptides and Protein Drug Delivery
- Green & Sustainable Pharma
- 2D & 3D Printing in Drug Delivery
- Pre-Formulation & Formulation Aspects
- Pharmacokinetics and Pharmacodynamics in drugs
- Routes of Drug Delivery
- Nanotechnology in Drug Delivery
- Global Drug Delivery Policy
- Biomedicine and Pharmacotherapy
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | March 19-21, 2018 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | Day 3 |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Pharmaceutics & Drug Delivery Research
- Journal of Nanomedicine & Nanotechnology: Open Access
- Journal of Pharmaceutical Sciences & Emerging Drugs
Abstracts will be provided with Digital Object Identifier by














































