Theme: Challenges and Opportunities in Transforming the Pharma Industry
Pharmaceutica 2019
Track 1: Bio-Pharmaceutics
Bio Pharmaceutics conferences plays an important role in drug discovery like drug disposition, Innovations in clinical development, Pharmaceutical technology, Pharmaceutics and drug delivery, Drug design, Targeted drug, gene delivery, Sustained drug delivery system, Routes of administration, Fundamental drug development.
For more information visit: Pharmaceutical Conferences
Related Bio Pharmaceutics Conferences: Bio Pharmaceutics events | Bio Pharmaceutics meetings | Bio pharmaceutics 2018 | Pharmaceutics 2018 | Pharmaceutical Biotechnology Conferences | Pharma Biotech Meetings | Pharmaceutics 2019 Conferences | Pharmaceutics 2019 | Euro Pharmaceutics 2018 | Euro Bio Pharmaceutics | Euro Bio Pharmaceutics 2018 | Bio Pharma Conferences 2018 | Bio Pharma Experts Meetings | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Bio Pharma Societies in USA: International Biopharmaceutical Association, Consumer Healthcare Products Association (CHPA), Generic Pharmaceutical Association (GPhA), International Pharmaceutical Expedient Council, Pharmaceutical Research and Manufacturers of America (PhRMA) Synthetic Organic Chemical Manufacturers Association (SOCMA), Biotech Industry Association (BIO), Parenteral Drug Association (PDA), Chinese Biopharmaceutical Association, Pharma & Biopharma Outsourcing Association (PBOA), SAFE-BioPharma Association and The BioPharmaceutical Emerging Best Practices Association (BEBPA)
Bio Pharma Societies in Europe: European Federation of Pharmaceutical Industries and Associations (EFPIA), European Biopharmaceutical Enterprises (EBE), European Association of Pharma Biotechnology, International Federation of Pharmaceutical Manufacturers & Associations, European Federation of Biotechnology, EuropaBio and European and Developing Countries Clinical Trials Partnership
Bio Pharma Societies in Asia: The Hong Kong Association of the Pharmaceutical Industry, International Pharmaceutical Manufacturers Group, International Research-based Pharmaceutical Manufacturers Association, Japan Pharmaceutical Manufacturers Association, Korea Pharmaceutical and Bio-Pharma Manufacturers Association, Korean Research-based Pharmaceutical Industry Association, Organization of Pharmaceutical Producers of India, Pharmaceutical Association of Malaysia, Pharmaceutical and Healthcare Association of the Philippines, China Pharmaceutical Innovation & Research Development Association, Pharmaceutical Research & Manufacturers Association, R&D-based Pharmaceutical Association Committee, Singapore Association of Pharmaceutical Industries
Bio Pharmaceutics Journals: European Journal of Pharmaceutics and Biopharmaceutics, International Journal of Biopharmaceutics, Journal of Biopharmaceutics and Biotechnology, Biopharmaceutics and Drug Disposition, Journal of Pharmacokinetics and Biopharmaceutics, Journal of Biopharmaceutical Statistics, Journal of Biopharmaceutics and Therapeutic Challenges, Journal of applied biopharmaceutics and pharmacokinetics, Journal of Applied Biopharmaceutics and Pharmacokinetics, Statistics in Biopharmaceutical Research, EBR - European Biopharmaceutical Review, Russian Journal of Biopharmaceuticals
Track 2: Pharmaceutical Chemistry
Pharmaceutical Chemistry is the study of drug design to optimize pharmacokinetics and pharmacodynamics, and synthesis of new drug molecules (Medicinal Chemistry 2019). Pharmaceutical Chemistry is a branch of chemistry that deals with the chemical, biochemical and pharmacological aspects of drugs. It includes synthesis/isolation, identification, structural elucidation, structural modification, structural activity relationship (SAR) studies, study of the chemical characteristics, biochemical changes after drug administration and their pharmacological effects as well as analysis of drugs. In more simple words it is more broader than medicinal chemistry in its application also in the fields of analysis, identification, as well as, structural elucidation of drugs
For more information visit: Pharmaceutical Conferences
Related Pharmaceutical Chemistry Conferences: Pharmaceutical Chemistry Symposiums | Pharmaceutical Chemistry Conferences | Pharmaceutical Chemistry Meetings | Medicinal Chemistry Conferences | Pharmaceutical Chemistry Congress | Pharmaceutica Conferences | Pharma Conferences | Pharma Medicinal Chemistry 2018 | Medicinal Chemistry Events | Pharmaceutica Meetings | Medicinal Chemistry Meetings | Medicinal Chemistry Congress | Pharmaceutical Chemistry Conferences 2018 | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Pharmaceutical Chemistry Societies in USA: American Chemical Society, American Association of Pharmaceutical Scientists, Medicinal Chemistry Society, American Chemistry Council (ACC), International Council of Chemical Associations, Chemical Institute of Canada (CIC) and World Association of Theoretical and Computational Chemists (WATOC)
Pharmaceutical Chemistry Societies in Europe: EuCheMS (European Association for Chemical and Molecular Sciences), Chemical Industries Association, European Federation for Medicinal Chemistry (EFMC), Swiss Chemical Society (SCS), Turkish Association of Medicinal and Pharmaceutical Chemistry, Royal Society of Chemistry (RSC), Slovenian Pharmaceutical Society, Polish Society of Medicinal Chemistry, Hungarian Chemical Society (HCS), The French Medicinal Chemistry Society (SCT) and Turkish Chemical Society (TCS)
Pharmaceutical Chemistry Societies in Asia and Pacific: The Chemical Society of Japan, Swiss Chemical Society, The Israel Chemical Society, Indian Society of Chemists and Biologists, The Royal Australian Chemical Institute, Federation of Asian Chemical Societies (FACS), Chinese Chemical Society, Korean Chemical Society, Singapore National Institute of Chemistry and Chemical Research Society of India
Pharmaceutical Chemistry Journals: Pharmaceutical Chemistry Journal, Chemical Sciences Journal, International Journal of Pharmaceutical Chemistry, Journal of the American Chemical Society, International Journal of Medicinal Chemistry, International Journal of Pharmaceutical Chemistry and Analysis, Journal of Pharmaceutical Chemistry, European Journal of Medicinal Chemistry and Journal of Pharmaceutical Chemistry & Chemical Science
Track 3: Drug Targeting and Design
Drug Design Conferences, often referred to as rational drug design or simply rational design, is the inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic small molecule that activates or inhibits the function of a biomolecule such as a protein, which in turn results in a therapeutic benefit to the patient. In the most basic sense, drug design involves the design of molecules that are complementary in shape and charge to the biomolecular target with which they interact and therefore will bind to it. Drug design frequently but not necessarily relies on computer modeling techniques. This type of modeling is sometimes referred to as computer-aided drug design. Finally, drug design that relies on the knowledge of the three-dimensional structure of the biomolecular target is known as structure-based drug design. In addition to small molecules, biopharmaceuticals and especially therapeutic antibodies are an increasingly important class of drugs and computational methods for improving the affinity, selectivity, and stability of this protein-based therapeutics have also been developed
For more information visit: Pharmaceutical Conferences
Related Drug Design Conferences: Drug Discovery Conferences | Drug Discovery Congress | Drug Discovery Symposiums | Drug Development Conferences | Drug Discovery Meetings | Drug Design Conferences | Drug Designing Conferences | Drug Designing Meetings | Drug Safety Conferences | Drug Discovery Congress 2018 | Drug Discovery 2018 | Pharmaceutica 2019 | NDDS Conferences | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Drug Discovery Societies in USA: American Association of Pharmaceutical Scientists (AAPS), American Peptide Society (APS), American Society for Biochemistry and Molecular Biology (ASBMB), American Society for Pharmacology and Experimental Therapeutics (ASPET), Biophysical Society, American Association for Clinical Chemistry (AACC), American Crystallographic Association (ACA), American Institute of Chemical Engineers (AIChE)
Drug Discovery Societies in Europe: Royal Society of Biology, Association of the British Pharmaceutical Industry (ABPI), The BioIndustry Association, British Pharmacological Society (BPS), Biochemical Society, The Physiological Society, International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), European Photochemistry Association, European Federation for Medicinal Chemistry (EFMC), European Federation for Pharmaceutical Sciences (EUFEPS), European Federation for the Science and Technology of Lipids (EuroFedLipid), Euroscience and International Pharmaceutical Federation
Drug Discovery Societies in Asia and Pacific: Chemical Society of Thailand, Singapore National Institute of Chemistry, Serbian Chemical Society (SCS), Integrated Chemists of the Philippines, New Zealand Institute of Chemistry, Malaysian Institute of Chemistry, Association of Latvian Chemical and Pharmaceutical Industry (LAKIFA), Kuwait Chemical Society
Drug Discovery Journals: American Journal of Drug Discovery and Development, Nature Reviews Drug Discovery, Drug Discovery Today, Expert Opinion on Drug Discovery, Current Pharmaceutical Design, Current Drug Discovery Technologies, International Journal of Drug Discovery, Drug Metabolism and Pharmacokinetics, International Journal of Drug Development and Research (IJDDR) and Drug Development Research, Journal of Computer-Aided Molecular Design, Drug Design, Development and Therapy, Journal of Drug Discovery, Development and Delivery, The Open Drug Discovery Journal and Drug Development and Industrial Pharmacy
Track 4: Pharmacokinetics and Pharmacodynamics in Drugs
Pharmacokinetics is currently defined as the study of the time course of drug absorption, distribution, metabolism, and excretion. Clinical pharmacokinetics is the application of pharmacokinetic principles to the safe and effective therapeutic management of drugs in an individual patient. Primary goals of clinical pharmacokinetics include enhancing efficacy and decreasing toxicity of a patient’s drug therapy. The development of strong correlations between drug concentrations and their pharmacologic responses has enabled clinicians to apply pharmacokinetic principles to actual patient situations.
Pharmacodynamics refers to the relationship between drug concentration at the site of action and the resulting effect, including the time course and intensity of therapeutic and adverse effects. The effect of a drug present at the site of action is determined by that drug’s binding with a receptor.
For more information visit: Pharmaceutical Conferences
Related Pharmacokinetics Conferences: Pharmacokinetics Conferences | Pharmacokinetics and Pharmacodynamics Conferences | Conference on Pharmacokinetics | Annual Pharmacokinetics Conferences | Pharmaceutical Conferences | Pharmaceutical Meetings | Pharmaceutical Exhibitions | Pharmaceutical Events | Pharmaceutical Conferences Europe | Pharmaceutical Conferences Asia | Pharmaceutical Conferences USA | Euro Pharmaceutics 2018 | Pharmaceutics 2018 | Pharmaceutics Conferences 2018 | Pharmaceutics Conference 2018 | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Pharmacokinetics Societies in USA: AACP (American Association of Colleges in Pharmacy), American Association of Pharmaceutical Scientists-AAPS, American College of Clinical Pharmacy (ACCP), ACPE – Accreditation Council for Pharmacy Education, AMCP (Academy of Managed Care Pharmacy), American Pharmacists Association (APhA), American Society of Consultant Pharmacists -ASCP, ASHP – American Society of Health-System Pharmacists, AZO (Alpha Zeta Omega), BPS (Board of Pharmacy Specialties), CPA – Connecticut Pharmacists Association
Pharmacokinetics Societies in Europe: European Association of Employed Community Pharmacists in Europe (EPhEU), Pharmaceutical Group of the European Union (PGEU), Danish Association of Pharmaconomists, Pharmaceutical Society of Ireland, National Pharmacy Association, Pharmaceutical Society of Northern Ireland and Royal Pharmaceutical Society (RPS), International Pharmaceutical Federation (FIP) and International Pharmaceutical Students' Federation (IPSF)
Pharmacokinetics Societies in Asia and Middle East: Australian College of Pharmacy, Pharmaceutical Society of Australia, The Pharmacy Guild of Australia, The Society of Hospital Pharmacists of Australia, Chinese Pharmaceutical Association, The Pharmaceutical Association of Israel and Kuwait Pharmaceutical Association
Pharmacokinetics & Pharmacodynamics Journals: Drug Metabolism and Pharmacokinetics, Journal of Pharmacokinetics and Pharmacodynamics, Journal of Pharmacokinetics & Experimental Therapeutics, Clinical Pharmacokinetics and International Journal of Pharmacokinetics
Track 5: Pharmaceutical Formulation
Pharmaceutics is the study of relationships between preformulation, pharmaceutical formulation, delivery, disposition and clinical response. The inherent instability nature of a new drug will alter its desired form into undesired form when presented in a suitable dosage form with the excipient/s upon storage. In early days this process was confined only for assessing few characteristics, but today this process is being considered as a formulation strategy and hence tremendous technological advancement has been achieved in this field which enables us to save time and money through planned management system and hence impacts Pharmaceutica 2019 to be a formulation conference. Use of glorious statistical software even based on artificial neural networking are made the task of preformulation and optimization process easier. Role of preformulation studies techniques like freeze drying aspects projects the event Pharmaceutics 2019 to pose as a freeze drying meeting in drug discovery, drug development plays major role in pharmaceutical formulation development and the studies will help in different dosage forms design. With the increasing number of novel and specialized compounds being developed, a "one size fits all" approach to drug formulation and delivery is no longer optimal, necessitating the consideration of formulations unique to each drug. NDDS conference will discuss on Early Approaches, Present Scenario and Future Prospects of Preformulation events. There are more than 1400 sustained or controlled release drugs have been approved all over the world. Pharmaceutical conferences discuss the state-of-art technology being applied and involve advances in formulation studies.
For more information visit: Pharmaceutical Conferences
Related Pharmaceutical Fomulations Conferences: Pharmaceutical Formulations | Pharmaceutical Formulations Conferences | Pharmaceutical Formulations Meetings | Pharmaceutical Formulations Congress | Formulations 2019 | Pharmaceutical Sciences Conferences | Pharmaceutical Sciences Meetings | Pharmaceutical Sciences Exhibitions | Pharmaceutical Sciences Events | Pharmaceutical Sciences Conferences Europe | Pharmaceutical Sciences Conferences Asia | Pharmaceutical Sciences Conferences USA | Pharmaceutical Sciences Conferences Middle East | Pharmaceutical Sciences Conferences Middle East | Pharmaceutical Sciences 2019 | Pharmaceutical Sciences USA | Pharma Europe 2019 | Pharma Middle East 2019 | Pharmaceutical Sciences Conferences 2019 | Pharma Research 2019 | Pharma Industry Conferences 2019 | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Pharmaceutical Formulations Societies in USA: International Society for Pharmaceutical Engineering, Consumer Healthcare Products Association (CHPA), Drug Information Association (DIA), Generic Pharmaceutical Association (GPhA), Pharmaceutical Research and Manufacturers of America (PhRMA), American Association of Pharmaceutical Scientists, American Pharmacists Association, American Society of Health Systems Pharmacists, National Association of Boards of Pharmacy, National Community Pharmacists Association, National Association of Chain Drug Stores (NACDS) and Pharmaceutical Care Management Association (PCMA)
Pharmaceutical Formulations Societies in Europe: International Federation of Pharmaceutical Manufacturers & Associations IFPMA, Association of the British Pharmaceutical Industry, European Federation of Pharmaceutical Industries and Associations (EFPIA)
Pharmaceutical Formulations Societies in Asia and Middle East: Pharmaceutical Society of Australia, The Japan Pharmaceutical Manufacturers Association (JPMA)
Pharmaceutical Formulations Journals: International Journal of Pharmaceutical Formulation and Analysis, International Journal of Drug Formulation and Research, International Journal of Drug Formulation & Research, Journal of Pharmaceutical Sciences, Pharmaceutical Development and Technology, International Journal of Pharmaceutical Formulation and Analysis, Pharmaceutical Research, Journal of Formulation Science & Bioavailability and International Journal of Pharmaceutics
Track 6: Pharmaceutical Manufacturing
Drug manufacturing is the process of industrial-scale synthesis of pharmaceutical drugs by pharmaceutical companies. The process of drug manufacturing can be broken down into a series of unit operations, such as milling, granulation, coating, tablet pressing, and others
For more information visit: Pharmaceutical Conferences
Related Pharmaceutical Manufacturing Conferences: Pharmaceutical Manufacturing Conferences | Drug Manufacturing Conferences | Drug Manufacturing events | Pharmaceutical Manufacturing Events | Pharmaceutical Manufacturing Meetings | Pharmaceutical Formulations | Pharma Conferences | Pharma Meetings | Pharma Exhibitions | Pharma Events | Pharmaceutical Conferences Europe | Pharmaceutical Conferences Asia | Pharmaceutical Conferences USA | Pharmaceutical Conferences Middle East | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Pharmaceutical Manufacturing Societies in USA: Pharmaceutical Research and Manufacturers of America, Generic Pharmaceutical Association (GPhA), US Food and Drug Administration, The Latin American Federation of Pharmaceutical Industry, Consumer Healthcare Products Association (CHPA) and Drug Information Association (DIA)
Pharmaceutical Manufacturing Societies in Europe: Association of the British Pharmaceutical Industry (ABPI), The International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), Association of Research-Based Pharmaceutical Companies (AIFD), European Federation of Pharmaceutical Industries and Associations (EFPIA), EuropaBio, European Biological Enterprises (EBE), Association of International Pharm Manufacturers (AIPM),
Pharmaceutical Manufacturing Societies in Asia and Pacific: The Hong Kong Association of the Pharmaceutical Industry, Organization of Pharmaceutical Producers of India (OPPI), China Pharmaceutical Industry Association (CPIA), Chinese Pharmaceutical Association, R&D-based Pharmaceutical Association Committee (RDPAC), Pharmaceutical Association of Malaysia, European Federation of Pharmaceutical Industries and Associations (EFPIA), Singapore Association of Pharmaceutical Industries, Innovative Pharmaceutical Association South Africa (IPASA), South African Association of Pharmacists in Industry (SAAPI), Korean Research-Based Pharmaceutical Industry Association (KRPIA) International Research-Based Pharmaceutical Manufacturers Association (IRPMA), Pharmaceutical Research and Manufacturers Association (PreMA)
Pharmaceutical Manufacturing Journals: Journal of Pharmaceutical Innovation, Journal of Pharmaceutical Sciences, Pharma Manufacturing, The Pharmaceutical Journal, World Pharma Today and European Pharmaceutical Manufacturer
Track 7: Pharmaceutical Nanotechnology
Size reduction is a fundamental unit operation having important applications in pharmacy. It helps in improving solubility and bioavailability, reducing toxicity, enhancing release and providing better formulation opportunities for drugs. In most of the cases, size reduction is limited to micron size range, for example, various pharmaceutical dosage forms like powder, emulsion, suspension etc. Drugs in the nanometer size range enhance performance in a variety of dosage forms. Major advantages of nanosizing include (i) increased surface area, (ii) enhanced solubility, (iii) increased rate of dissolution, (iv) increased oral bioavailability, (v) more rapid onset of therapeutic action, (vi) less amount of dose required, (vii) decreased fed/fasted variability, and (viii) decreased patient-to-patient variability.
Pharmaceutical nanotechnology has provided more fine-tuned diagnosis and focused treatment of disease at a molecular level. Pharmaceutical nanotechnology is most innovative and highly specialized field, which will revolutionize the pharmaceutical industry in near future. Pharmaceutical nanotechnology presents revolutionary opportunities to fight against many diseases. It helps in detecting the antigen associated with diseases such as cancer, diabetes mellitus, neurodegenerative diseases, as well as detecting the microorganisms and viruses associated with infections. It is expected that in next 10 years market will be flooded with nanotechnology devised medicine.
For more information visit: Pharmaceutical Conferences
Related Pharmaceutical Nanotechnology Conferences: Pharmaceutical Nanotechnology Symposium | Pharmaceutical Nanotechnology Conferences | Pharmaceutical Nanotechnology Meetings | Pharmaceutical Nanotechnology Congress | Nano Medicine Meetings | Nano Medicine Conferences | Nano Medicine Events | Nano Pharma Conferences | NanoMed Conferences | Nano Drug Delivery Conferences | Nanotech Conferences | Nano science Conferences | Nano Biotech Conferences | Nano Drug Delivery Meetings | Nanotechnology Conferences | Nanotechnology Meetings | Advanced Nanotechnology Conferences | Nanomaterials Conferences | Nano Today Conference | Nanotechnology Conferences 2018 | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Pharmaceutical Nanotechnology Societies in USA: International Association of Nanotechnology (IANT), American Bar Association Section Nanotechnology Project, American Chemical Society, American National Standards Institute Nanotechnology Panel, The American Society for Precision Engineering (ASPE), Converging Technologies Bar Association, National Institute for Nanotechnology, National Nanotechnology Initiative, International Institute for Nanotechnology
Pharmaceutical Nanotechnology Societies in Europe: European Nanoscience and Nanotechnology Association (ENNA), Erwin Schrödinger Society for Nanosciences (Austria), Nanotechnology Industries Association, British Society for Nanomedicine, Czech Nanotechnology Industries Association, European NanoBusiness Association (ENA), European Society for Precision Engineering and Nanotechnology, Russian Nanotechnology Corporation and Swiss Nanoscience Institute
Pharmaceutical Nanotechnology Societies in Asia and Pacific: Asian Nanoscience and Nanotechnology Association, National Center for Nanoscience and Technology, Iran Nanotechnology Initiative Council (INIC), Sri Lanka Institute of Nanotechnology and Nano Technology Research Association
Pharmaceutical Nanotechnology Journals: Pharmaceutical Nanotechnology, Journal of Pharmaceutics & Drug Delivery Research, Nano Today, International Journal of Pharmaceutical Sciences and Nanotechnology, International Journal of Medicine and Nanotechnology, Journal of Nanopharmaceutics and Drug Delivery and Journal of Nanotechnology
Track 8: Nano particulate Drug Delivery Systems
Nanoparticles (NPs) occur naturally and have been in existence for thousands of years as products of combustion and cooking of food. Nanomaterials differ significantly from other materials due to the following two major principal factors: the increased surface area and quantum effects. These factors can enhance properties such as reactivity, strength, electrical characteristics, and in vivo behavior. As the particle size decreases, a greater proportion of atoms are found at the surface compared to inside. An NP has a much greater surface area per unit mass compared with larger particles, leading to greater reactivity. In tandem with surface area effects, quantum effects can begin to dominate the properties of matter as size is reduced to the nanoscale. These can affect the optical, electrical, and magnetic behavior of materials. Their in vivo behavior can be from increased absorption to high toxicity of nanomaterials. New drug carrier systems are one can name soluble polymers, microparticles made of insoluble (or) biodegradable natural and synthetic polymers, microcapsules, cells, cell ghosts, lipoproteins, liposomes and micelles. Pharmaceutica 2019 evolves to be a drug disintegration conference, emulsion conference, capsule conference, and solubility conference. Pharmaceutical conferences will cover industry case studies, regulatory updates, latest therapies and technology innovations and much more.
Key players in the market include Amgen, Inc., AstraZeneca plc, Eli Lilly & Co., Ipsen S.A., Merck & Co., Novartis AG, Novo Nordisk A/S, Roche Holdings AG, Sanofi, Takeda Pharmaceutical Company Limited, and Teva Pharmaceutical Industries Limited. Leading API manufacturers include Bachem Holding AG, PolyPeptide Group, and Peptisyntha Inc. at the pharmaceutical companies’ conference
For more information visit: Pharmaceutical Conferences
Related Novel Drug Delivery Systems Conferences: Novel Drug Delivery Systems Symposium | Novel Drug Delivery Systems Conferences | Novel Drug Delivery Systems Meetings | Novel Drug Delivery Systems | Drug Delivery Conferences | Drug Delivery Conferences USA | Drug Delivery Conferences Europe | Drug Delivery Conferences Asia | Drug Delivery 2018 | Novel Drug Delivery | Nano Drug Delivery Conferences | Nano Drug Delivery | Novel Drug Delivery Experts Meeting | Drug Delivery and Novel Therapy Conferences | Pharmaceutics Conferences | Pharmaceutica Conferences | Drug Delivery Meetings | Drug Delivery and Formulation Conferences | Conference on Pharmaceutics and Drug Delivery Systems | Formulation and Drug Delivery Conference | NDDS Conference | Nanomedicine Conferences | Nano Pharma 2019 | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Novel Drug Delivery Systems Societies in USA: Drug Delivery Foundation, Controlled Release Society, Society for Biomaterials, Academy of Managed Care Pharmacy (AMCP), Accreditation Council for Pharmacy Education, American Association of Colleges of Pharmacy (AACP), American Association of Pharmaceutical Scientists (AAPS), American Association of Pharmacy Technicians (AAPT), American College of Clinical Pharmacy (ACCP)
Novel Drug Delivery Systems Societies in Europe: Austrian Association of Hospital Pharmacists, Austrian Pharmaceutical Society, British Pharmaceutical Nutrition Group, Deutsche Gesellschaft fur Geschichte der Pharmazie, Guild of Healthcare Pharmacists, International Pharmaceutical Federation (FIP), International Pharmaceutical Students' Federation, Royal Microscopial Society, Royal Pharmaceutical Society of Great Britain, Society of Hospital Pharmacists of Australia and Turkish Pharmacists Association
Novel Drug Delivery Systems Societies in Asia and Pacific: Cooperative Research Centres (CRC) Association, Kuwait Pharmaceutical Association, Malaysian Pharmaceutical Society, Pharmaceutical Society of Australia, Pharmaceutical Society of Korea, Pharmacy Guild of Australia and The Pharmaceutical Association of Mauritius
Novel Drug Delivery Systems Journals: Indian Journal of Novel Drug Delivery, Madridge Journal of Novel Drug Research (MJNDR), Journal of Drug Delivery, Journal of Nanopharmaceutics and Drug Delivery, Journal of Drug Delivery Science and Technology, Journal of Controlled Release, International Journal of Drug Delivery, Current Drug Delivery and Drug Delivery and Translational Research
Track 9: Smart Drug Delivery Systems
Smart drug delivery is a method of delivering medication to a patient in a manner that increases the concentration of the medication in some parts of the body relative to others. This means of delivery is largely founded on nanomedicine, which plans to employ nanoparticle-mediated drug delivery in order to combat the downfalls of conventional drug delivery. These nanoparticles would be loaded with drugs and targeted to specific parts of the body where there is solely diseased tissue, thereby avoiding interaction with healthy tissue. The goal of a targeted drug delivery systems is to prolong, localize, target and have a protected drug interaction with the diseased tissue. The conventional drug delivery system is the absorption of the drug across a biological membrane, whereas the targeted release system releases the drug in a dosage form. The advantages to the targeted release system is the reduction in the frequency of the dosages taken by the patient, having a more uniform effect of the drug, reduction of drug side-effects, and reduced fluctuation in circulating drug levels. The disadvantage of the system is high cost, which makes productivity more difficult and the reduced ability to adjust the dosages.
For more information visit: Pharmaceutical Conferences
Related Drug Delivery Conferences: Drug Delivery Conferences | Drug Delivery Conferences USA | Drug Delivery Conferences Europe | Drug Delivery Conferences Asia | Drug Delivery 2018 | Novel Drug Delivery | Nano Drug Delivery Conferences | Novel Drug Delivery Systems Conferences | Novel Drug Delivery Systems Meetings | NDDS Conferences | Pharmaceutica 2019 | Pharmaceutics 2018 | Euro Pharmaceutics 2018 | Pharmaceutics Asia | Bio Pharmaceutics conferences | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Drug Delivery Societies in USA: Parenteral Drug Association, National Association of Drug Diversion Investigators, Inc. (NADDI), Drug & Alcohol Testing Industry Association (DATIA), Association for Accessible Medicines (AAM), U.S. Food and Drug Administration, International Federation of Pharmaceutical Manufaturers & Associations, Drug Information Association, National Institute on Drug Abuse (NIDA), National Association of Drug Court Professionals, Consumer Healthcare Products Association
Drug Delivery Societies in Europe: Austrian Pharmaceutical Society, Croatian Pharmaceutical Society, Czech Pharmaceutical Society, European Pharmaceutical Students Association, FIGON - Netherlands Federation for Innovative Drug Research, Finnish Pharmaceutical Society, German Pharmaceutical Society (DPhG), Hellenic Pharmaceutical Society and Hungarian Society for Pharmaceutical Sciences (HSPS)
Drug Delivery Societies in Asia and Pacific: Indian Pharmaceutical Association, Indian Drug Manufacturers' Association and Bulk Drug Manufacturers Association India
Drug Delivery Journals: Journal of Drug Delivery, Journal of Pharmaceutics & Drug delivery Research, Journal of Drug Delivery Science and Technology, Advanced Drug Delivery Reviews, Drug Delivery, International Journal of Drug Delivery, Journal of Nanopharmaceutics and Drug Delivery, Drug Delivery and Translational Research, American Journal of Drug Delivery and Therapeutics, American Journal of Drug Delivery, Journal of Advanced Drug Delivery, International Journal of Drug Delivery Technology, Journal of Drug Delivery Research
Track 10: Nanomedicine and Biomedical Applications
Nanomedicine is simply the nanotechnology applications in a healthcare setting and the majority of benefits that have already been seen involve the use of nanoparticles to improve the behavior of drug substances and in drug delivery. Today, nanomedicine conferences are used globally to improve the treatments and lives of patients suffering from a range of disorders including ovarian and breast cancer, kidney disease, fungal infections, elevated cholesterol, menopausal symptoms, multiple sclerosis, chronic pain, asthma and emphysema. Nanomedicine has the potential to develop radical new therapies based on an unprecedented control over both intracellular processes and the extracellular environment at the nanometer scale. To create precise solutions for intricate medical challenges in the area of wound healing, tissue regeneration and mitochondrial disease physical scientists, medical doctors, and industrial partners, work closely in the Radboud Nanomedicine Alliance. The National Nanotechnology Initiative expects new commercial applications in the pharmaceutical industry that may include advanced delivery systems, new therapies, and in vivo imaging.
For more information visit: Pharmaceutical Conferences
Related Nanomedicine Conferences: Nanomedicine Congress | Nanomedicine Meetings | Nanomedicine Conferences | Nanomedicine Events | Nano Pharma Conferences | NanoMed Conferences | Nano Drug Delivery Conferences | Nanotech Conferences | Nano science Conferences | Nano Biotech Conferences | Nano Drug Delivery Meetings | Nanotechnology Conferences | Nanotechnology Meetings | Nanotechnology Congress | Conference on Nanotechnology | Nanotech Conferences | Nanobiotech Conferences | Nanoscience Congress | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Nanomedicine Societies in USA: American Academy of Nanomedicine, Nano Canadian Society, American Nano Society, American Society for Nanomedicine, Society for Personalized Nano Medicine, International Association of Nanotechnology and Graphene Stakeholders Association
Nanomedicine Societies in Europe: Nanotechnology Industries Association, Royal Microscopical Society, Royal Society - Nanotechnology and Nanoscience, British Society for Nanomedicine, European Society for Nanomedicine, French Society for Nanomedicine, European NanoBusiness Association, European Society for Precision Engineering and Nanotechnology, Czech Nanotechnology Industries Association and Erwin Schrödinger Society for Nanosciences
Nanomedicine Societies in Asia: National Center for Nanoscience and Technology, Indian Society for Nanomedicine, Nano Technology Research Association and Indian Society of Nanomedicine.
Nanomedicine Journals: Nanomedicine: Nanotechnology, Biology and Medicine, Journal of Nanomedicine & Biotherapeutic Discovery, Journal of Nanomedicine & Nanotechnology, Nanomedicine Journal (MJN), International Journal of Nanomedicine, European Journal of Nanomedicine, Nanomedicine and Nanobiotechnology, American Journal of Nanotechnology, Journal of Nanomedicine Research (JNMR), Journal of Nanotechnology, Nanotechnology, Science and Applications and Journal of Nanoparticle Research, Journal of Interdisciplinary Nanomedicine (JOIN)
Track 11: Biomaterials in Drug Delivery
A biomaterial is any substance that has been engineered to interact with biological systems for a medical purpose - either a therapeutic (treat, augment, repair or replace a tissue function of the body) or a diagnostic one. Biomaterials conferences can be derived either from nature or synthesized in the laboratory using a variety of chemical approaches utilizing metallic components, polymers, ceramics or composite materials. They are often used and/or adapted for a medical application, and thus comprise whole or part of a living structure or biomedical device which performs, augments, or replaces a natural function. Such functions may be benign, like being used for a heart valve, or may be bioactive with a more interactive functionality such as hydroxy-apatite coated hip implants. Biomaterials 2019 are also used every day in dental applications, surgery, and drug delivery. For example, a construct with impregnated pharmaceutical products can be placed into the body, which permits the prolonged release of a drug over an extended period of time. A biomaterial may also be an autograft, allograft or xenograft used as a transplant material.
For more information visit: Pharmaceutical Conferences
Related Biomaterials Conferences: Biomaterials Conferences | Biomaterial Meetings | European Conference on Biomaterials | Biomaterials Congress | Biomaterials events | Nanomaterials Conference | Nanomaterials Congress | Materials Science Conferences | Conferences on Material Science | Nano Today Conference | Nanomaterials events | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Biomaterials Societies in USA: Society for Biomaterials (SFB), The Canadian Biomaterials Society (CBS), American National Standards Institute Nanotechnology Panel (ANSI-NSP), National Nanotechnology Initiative, International Institute for Nanotechnology and Nanoscience and Nanotechnology Center
Biomaterials Societies in Europe: European Society for Biomaterials, Russian Nanotechnology Corporation, NanoNed, Nanotechnology Industries Association (NIA), International Iberian Nanotechnology Laboratory, Swiss Nanoscience Institute (SNI), London Centre for Nanotechnology and Romanian Society for Biomaterials
Biomaterials Societies in Asia and Middle East: International Union of Societies for Biomaterials Science and Engineering (IUSBSE), Chinese Society for Biomaterials (CSBM), Asian Polymer Association (APA), National Center for Nanoscience and Technology, National Institute for Nanotechnology, Australian Institute for Bioengineering and Nanotechnology, Egypt Nanotechnology Center, International Center for Materials Nanoarchitectonics, Korean Society for Biomaterials, Australasian Society for Biomaterials and Tissue Engineering and Society for Biomaterials & Artificial Organs
Biomaterials Journals: Biomaterials, Journal of Biomedical Materials Research, Acta Biomaterialia, Journal of Biotechnology & Biomaterials, International Journal of Biomaterials, Journal of Biomaterials Applications and Biomaterials Science
Track 12: Vaccine Drug Delivery Systems
Vaccine is a material that induces an immunologically mediated resistance to a disease but not necessarily an infection. Vaccines are generally composed of killed or attenuated organisms or subunits of organisms or DNA encoding antigenic proteins of pathogens. Sub-unit vaccines though exceptionally selective and specific in reacting with antibodies often fail to show such reactions in circumstances such as shifts in epitopic identification center of antibody and are poorly immunogenic. Delivery of antigens from oil-based adjuvants such as Freunds adjuvant lead to a reduction in the number of doses of vaccine to be administered but due to toxicity concerns like inductions of granulomas at the injection site, such adjuvants are not widely used. FDA approved adjuvants for human uses are aluminium hydroxide and aluminium phosphate in the form of alum. Hence, search for safer and potent adjuvants resulted in the formulations of antigen into delivery systems that administer antigen in particulate form rather than solution form.
Other reasons driving the development of vaccines as controlled drug delivery systems are as follows:
-
Immunization failure with conventional immunization regimen involving prime doses and booster doses, as patients neglect the latter. Vaccines delivery systems on the other hand:
-
Allow for the incorporation of doses of antigens so that booster doses are no longer necessary as antigens are released slowly in a controlled manner.
-
Control the spatial and temporal presentation of antigens to the immune system there by promoting their targeting straight to the immune cells.
For more information visit: Pharmaceutical Conferences
Related Drug Delivery Conferences: Drug Delivery Conferences | Drug Delivery Conferences USA | Drug Delivery Conferences Europe | Drug Delivery Conferences Asia | Drug Delivery 2018 | Novel Drug Delivery | Novel Drug Delivery Systems Conferences | Novel Drug Delivery Systems Meetings | Novel Drug Delivery Systems Congress | Nano Drug Delivery 2018 | Drug Delivery Conferences 2018 | Drug Delivery Events | Pharmaceutics and Novel Drug Delivery | Pharmaceutics Conferences 2018 | NDDS Conferences | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Drug Delivery Societies in USA: Parenteral Drug Association, National Association of Drug Diversion Investigators, Inc. (NADDI), Drug & Alcohol Testing Industry Association (DATIA), Association for Accessible Medicines (AAM), U.S. Food and Drug Administration, International Federation of Pharmaceutical Manufaturers & Associations, Drug Information Association, National Institute on Drug Abuse (NIDA), National Association of Drug Court Professionals, Consumer Healthcare Products Association
Drug Delivery Societies in Europe: Austrian Pharmaceutical Society, Croatian Pharmaceutical Society, Czech Pharmaceutical Society, European Pharmaceutical Students Association, FIGON - Netherlands Federation for Innovative Drug Research, Finnish Pharmaceutical Society, German Pharmaceutical Society (DPhG), Hellenic Pharmaceutical Society and Hungarian Society for Pharmaceutical Sciences (HSPS)
Drug Delivery Societies in Asia and Pacific: Indian Pharmaceutical Association, Indian Drug Manufacturers' Association and Bulk Drug Manufacturers Association India
Drug Delivery Journals: Journal of Drug Delivery, Journal of Pharmaceutics & Drug delivery Research, Journal of Drug Delivery Science and Technology, Advanced Drug Delivery Reviews, Drug Delivery, International Journal of Drug Delivery, Journal of Nanopharmaceutics and Drug Delivery, Drug Delivery and Translational Research, American Journal of Drug Delivery and Therapeutics, American Journal of Drug Delivery, Journal of Advanced Drug Delivery, International Journal of Drug Delivery Technology, Journal of Drug Delivery Research
Track 13: Medical Devices for Drug Delivery
In this session we will focus on medical devices designed for drug delivery conferences through the pulmonary and nasal routes. These routes are of interest for local delivery, as in asthma, but also for rapid delivery of drugs to the system circulation and direct delivery to the central nervous system. Devices that account for specific anatomical and physiological features of the intranasal and pulmonary routes will be featured. Drug delivery devices are specialized tools for the delivery of a drug or therapeutic agent via a specific route of administration. Such devices are used as part of one or more medical treatments. Many in the industry have long felt overly burdened by what they consider to be an unnecessarily complex approval process. Critics claim it impedes innovation and delays the availability of better health care. In order to help innovators bring health care to the public Pharmaceutica 2019 hosts drug delivery conferences throughout the year.
For more information visit: Pharmaceutical Conferences
Related Drug Delivery Conferences: Drug Delivery Conferences | Drug Delivery Conferences USA | Drug Delivery Conferences Europe | Drug Delivery Conferences Asia | Drug Delivery 2018 | Novel Drug Delivery | Novel Drug Delivery Systems Symposium | Novel Drug Delivery Systems Conferences | Novel Drug Delivery Systems Meetings | Novel Drug Delivery Systems Congress | | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Drug Delivery Societies in USA: Parenteral Drug Association, National Association of Drug Diversion Investigators, Inc. (NADDI), Drug & Alcohol Testing Industry Association (DATIA), Association for Accessible Medicines (AAM), U.S. Food and Drug Administration, International Federation of Pharmaceutical Manufaturers & Associations, Drug Information Association, National Institute on Drug Abuse (NIDA), National Association of Drug Court Professionals, Consumer Healthcare Products Association
Drug Delivery Societies in Europe: Austrian Pharmaceutical Society, Croatian Pharmaceutical Society, Czech Pharmaceutical Society, European Pharmaceutical Students Association, FIGON - Netherlands Federation for Innovative Drug Research, Finnish Pharmaceutical Society, German Pharmaceutical Society (DPhG), Hellenic Pharmaceutical Society and Hungarian Society for Pharmaceutical Sciences (HSPS)
Drug Delivery Societies in Asia and Pacific: Indian Pharmaceutical Association, Indian Drug Manufacturers' Association and Bulk Drug Manufacturers Association India
Drug Delivery Journals: Journal of Drug Delivery, Journal of Pharmaceutics & Drug delivery Research, Journal of Drug Delivery Science and Technology, Advanced Drug Delivery Reviews, Drug Delivery, International Journal of Drug Delivery, Journal of Nanopharmaceutics and Drug Delivery, Drug Delivery and Translational Research, American Journal of Drug Delivery and Therapeutics, American Journal of Drug Delivery, Journal of Advanced Drug Delivery, International Journal of Drug Delivery Technology, Journal of Drug Delivery Research
Track 14: Biologics & Biosimilars:
Biologics are medicines made from living cells through highly complex manufacturing processes and must be handled and administered under carefully monitored conditions. Biologics are used to prevent, treat, diagnose, or cure a variety of diseases including cancer, chronic kidney disease, autoimmune disorders, and infectious diseases. Euro Biosimilars is exactly what its name implies — it is a biologic that is “similar” to another biologic drug already approved by the FDA. Under U.S. law, a biosimilar is approved based on a showing that it is “highly similar” to an FDA-approved biological product, known as a reference product. It may not have any clinically meaningful differences in terms of safety and effectiveness from the reference product.
For more information visit: Pharmaceutical Conferences
Related Biosimilars Conferences: Biosimilars Conferences | Biologics Conferences | Euro Biosimilars Conferences | Euro Biosimilars 2019 | Biosimilars USA | Bioequivalence Conferences | Bioavailability Congress | Bio waivers Congress | Bioavailability Meetings | Biosimilars Conferences | Biologics conferences | Euro Biosimilars | Biosimilars USA | Generic Medicine Meetings | Generic Drug Conferences | Generic Pharma Conferences | Generic Pharma Congress | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Biosimilars Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP) and Canadian Society of Intestinal research (CSIR)
Biosimilars Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF) and Association of the British Pharmaceutical Industry (ABPI)
Biosimilars Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA) and Kuwait Pharmaceutical Association (KPA).
Biosimilars Journals: Biosimilars, Generics and Biosimilars Initiative Journal, Journal of Generic Medicines, Progress in Biologics & Biosimilars Research (PBBR) and Journal of Biopharmaceutical Statistics
Track 15: Pharmaceutical Analysis
Pharmaceutical analysis 2018 is a process or a sequence of processes to identify and/or quantify a substance or drug, the components of a pharmaceutical solution or mixture or the determination of the structures of chemical compounds used in the formulations 2018 of pharmaceutical product. Analytical techniques conferences mainly used for the separation of the components from the mixture and for the determination of the structure of the compounds.
For more information visit: Pharmaceutical Conferences
Related Pharmaceutical Analysis Conferences: Pharmaceutical Analysis Events | Analysis Conferences | Analytical Conferences | Pharmaceutical Analysis Meetings | Pharmaceutical Analysis Congress | Analytical chemistry Conferences | Bioequivalence Conferences | Bioavailability Congress | Bio waivers Congress | Bioavailability Meetings | Biosimilars Conferences | Biologics conferences | Euro Biosimilars | Biosimilars USA | Generic Medicine Meetings | Generic Drug Conferences | Generic Pharma Conferences | Generic Pharma Congress | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Pharmaceutical Analysis Societies in USA: American Chemical Society, American Society for Mass Spectrometry, Association of Analytical Chemists (ANACHEM)
Pharmaceutical Analysis Societies in Europe: Society for Analytical Chemistry, Joint Pharmaceutical Analysis Group (JPAG)
Pharmaceutical Analysis Societies in Asia: Austrian Society of Analytical Chemistry, The Japan Society for Analytical Chemistry (JSAC), The Spectroscopical Society of Japan (SPSJ), Indian Society of Analytical Scientists (ISAS)
Pharmaceutical Analysis Journals: Journal of Pharmaceutical Analysis, Pharmaceutica Analytica Acta, Research & Reviews: Journal of Pharmaceutical Analysis, Current Pharmaceutical Analysis and Analytical Chemistry
Track 16: Pharmaceutical Process Validation
Quality is always an imperative prerequisite when we consider any product. Therefore, drugs must be manufactured to the highest quality levels. End-product testing by itself does not guarantee the quality of the product. Quality assurance techniques must be used to build the quality into the product at every step and not just tested for at the end. In pharmaceutical industry 2019, Process Validation performs this task to build the quality into the product because according to ISO 9000:2000, it had proven to be an important tool for quality management of pharmaceuticals. Validation is one of the important steps in achieving and maintaining the quality of the final product. If each step of production process is validated we can assure that the final product is of the best quality. Validation of the individual steps of the processes is called the process validation. Different dosage forms have different validation protocols. Process Validation is one of the important steps in achieving and maintaining the quality of final product. It gives a higher degree of assurance.
For more information visit: Pharmaceutical Conferences
Related Pharmaceutical Conferences: Pharmaceutical Events | Pharmaceutical Meetings | Pharma Conferences | World Pharma Conferences | World Pharma Meetings | Pharma Congress | Pharma Congress 2018 | Pharma Europe 2018 | Pharma Meetings | Pharma Events | Pharma Middle East Conferences | Pharmaceutics Conferences | Euro Pharmaceutics | Euro Bio Pharmaceutics | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Pharmaceutical Societies in USA: American Association of Colleges of Pharmacy (AACP), American Pharmacists Association (APhA), American Society for Pharmacy Law American Society of Consultant Pharmacists (ASCP), American Society of Health-System Pharmacists (ASHP), Professional Compounding Centers of America.
Pharmaceutical Societies in Europe: European Association of Employed Community Pharmacists in Europe (EPhEU), European Pharmaceutical Union (EPU), Pharmaceutical Group of the European Union (PGEU), Norwegian Pharmacy Association.
Pharmaceutical Societies in Asia: Australian College of Pharmacy, Pharmaceutical Society of Australia, The Pharmacy Guild of Australia, The Society of Hospital Pharmacists of Australia, Chinese Pharmaceutical Association.
Pharmaceutical Journals: Journal of Pharmaceutical Sciences, European Journal of Pharmaceutical Sciences, European Journal of Pharmaceutics and Biopharmaceutics, Indian Journal of Pharmaceutical Sciences, International Journal of Clinical Pharmacy, International Journal of Pharmaceutics, Journal of Pharmacy and Pharmaceutical Sciences, Journal of Pharmacy Practice and Research, The Pharmaceutical Journal and Scientia Pharmaceutica
Track 17: Pharmaceutical Packaging
Packaging is one of the largest industry sectors in the world, worth several billions. Pharmaceutical packaging represents a meager percentage of this colossal market. The global healthcare industry has seen a shift in paradigm and is now skewed toward effective and meaningful packaging. Packaging was considered as an afterthought which was required merely in the final stages of manufacturing for many pharmaceutical companies about a decade ago.
For more information visit: Pharmaceutical Conferences
Related Pharmaceutical Conferences: Pharmaceutical Packaging Events | Pharmaceutical Packaging Conferences | Pharmaceutical Conferences | Pharma Meetings | Pharma Congress | Pharmaceutics 2018 | Euro Pharmaceutics 2018 | Pharmaceutica Conferences | Pharma Industry Conference | Pharma Research Conferences | Pharmaceutical Sciences 2019 | Pharmaceutics Conferences | Pharmaceutics Meetings | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Pharmaceutical Societies in USA: American Coatings Association, Parenteral Drug Association (PDA), American Association of Pharmaceutical Scientists (AAPS), Consumer Healthcare Products Association, American Urological Association (AUA), Pharmacy Society of Wisconsin (PSW),
Pharmaceutical Societies in Europe: Pharmaceutical Society of New Zealand Incorporated (PSNZI), Royal Pharmaceutical Society (RPS), Association of British Pharmaceutical Industry (ABPI)
Pharmaceutical Societies in Asia and Pacific: Bangladesh Pharmaceutical Society (BPS), Society for Pharmaceutical Dissolution Science (SPDC), Pharmaceutical and Bioscience Society (PBS)
Pharmaceutical Packaging Journals: The Pharmaceutical Journal, International Journal of Pharmacy and Pharmaceutical Sciences, International Journal of Pharmacy and Pharmaceutical Sciences, Journal of Medical Marketing
Track 18: Clinical Trials and Clinical Research
Clinical research 2018 aims to advance medical knowledge by studying people, either through direct interaction or through the collection and analysis of blood, tissues, or other samples.
A clinical trial involves research participants. It follows a pre-defined plan or protocol to evaluate the effects of a medical or behavioral intervention on health outcomes. By taking part in clinical trials conferences, participants not only play a more active role in their own health care, but they also can access experimental treatments and help others by contributing to medical research.
For more information visit: Pharmaceutical Conferences
Related Clinical Trial Conferences: Clinical Research Events | Clinical Research 2018 Conferences | Clinical Research Conferences | Clinical Trials Conferences | Clinical Research Meetings | Clinical Research Congress | Clinical Trials Meetings | Clinical Trials Congress | Clinical Events | Clinical trials Exhibitions | Clinical Research Exhibitions | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Clinical Research Societies in USA: Academy of Physicians in Clinical Research (APCR), American Association for Cancer Research (AACR), American Federation for Medical Research (AFMR), American Statistical Association (ASA), American Society for Clinical Investigation (ASCI).
Clinical Research Societies in Europe: Association of Clinical Research Organizations in the Netherlands (ACRON), Association for Clinical Data Management (ACDM), Association of CROs Czech Republic (ACRO-CZ), European Clinical Research Infrastructures Network (ECRIN), European Society for Clinical Investigation (ESCI).
Clinical Research Societies in Asia: Indian Council of Medical Research (ICMR), Japan CRO Association (JCROA), Pan-Asian Clinical Research Association (PACRA), Central Society for Clinical and Translational Research (CSCTR), Indian Society for Clinical Research (ISCR).
Clinical Research Journals: Clinical Trials, Contemporary Clinical Trials, Journal of Clinical Trials, Journal of Clinical Research & Bioethics, International Journal of Clinical Trials, Trials, International Journal of Open Access Clinical Trials, SM Journal of Clinical Trials, Clinical Research and Trials, American Journal of Clinical Trials (AJCT), Applied Clinical Research, Clinical Trials and Regulatory Affairs, International Journal of Medical and Clinical Research and HIV Clinical Trials
Track 19: Pharmacogenetics and Genomics
Pharmacogenetics is the science that supports understanding the part that a person's hereditary make-up plays in how well a prescription functions, and additionally what symptoms are probably going to happen, enhancing our capacity to distinguish the hereditary reasons for illnesses and look for new medication targets. Pharmacogenetics alludes to hereditary contrasts in metabolic pathways which can influence singular reactions to drugs, both as far as restorative impact and additionally antagonistic impacts.
Pharmacogenomics is a quickly creating field that has essential ramifications in individualized treatment for patients and its suggestion influence tranquilize advancement issues such as medication safety, efficiency, and customized health care. Pharmacogenomics consolidates customary pharmaceutical sciences 2019, for example, natural chemistry with explained colleague of qualities, proteins, and single nucleotide polymorphisms.
For more information visit: Pharmaceutical Conferences
Related Pharmacogenomics Conferences: Pharmacogenomics Events | Pharmacodynamics Conferences | Pharmaceutical Events | Pharmaceutical Conferences | Pharmacogenetics Conferences | Genomics Meetings | Pharmacogenetics Congress | Pharmaceutics Conferences | Pharmaceutica Conferences | Novel Drug Delivery Systems Conferences | Drug Delivery Conferences | Pharmaceutica Meetings | Euro Pharmaceutics | Euro Bio Pharmaceutics | | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Pharmacogenomics Societies in USA: American Society of Gene Therapy, Biotechnology and Biological Research Council, Genetics Society of America, The American Society of Human Genetics, Center for Genetics and Society, National Society of Genetic Counselors and American Society for Cell Biology.
Pharmacogenomics Societies in Europe: European Society of Human Genetics, European Peptide Society, European Federation of Biotechnology, European Association of Pharma Biotechnology, German Resource Centre for Genome Research and European federation of biotechnology
Pharmacogenomics Societies in Asia and Pacific: Asia Pacific Society of Human Genetics, Asia-Pacific Society of Eye Genetics, Asia Pacific Society of Human Genetics and Genetics Society of Thailand.
Pharmacogenomics Journals: Journal of Pharmacogenomics & Pharmacoproteomics, The Pharmacogenomics Journal, Pharmacogenomics, Current Pharmacogenomics and Personalized Medicine and Pharmacogenetics and Genomics
Track 20: Pharmaceutical Regulatory Affairs and Intellectual Property Rights
Regulatory Affairs conferences contribute essentially to the overall success of drug development, both at early pre-marketing stages and at all times post-marketing. The pharmaceutical industry 2019 deals with an increasing number of interesting drug candidates, all of which necessitate the involvement of the quality assurance in regulatory affairs department. The importance of intellectual property law is well established at all levels-statutory, administrative and judicial. It lays down minimum standards for protection and enforcement in member countries which are required to promote effective and adequate protection of intellectual property rights with a view to reducing distortions and impediments to international trade. The Agreement provides norms and standards in respect of following areas of intellectual properties are Patents, Trademarks, copyrights, Geographical indications, Industrial designs.
For more information visit: Pharmaceutical Conferences
Related Regulatory Affairs Conferences: Pharmaceutical Regulatory Affairs Conferences | Pharmaceutical Conferences | Pharmacology Conferences | Pharmacology Meetings | Pharmacology Congress | World Pharma Congress | Pharma Europe 2019 | Annual Pharma Conferences | Pharma Research Conferences | Pharma Market Research Conferences | European Pharma Conference | European Pharma Congress | Pharma Europe Conferences | Pharma Conferences USA | Pharma Conferences Europe | Pharma Conferences Middle east | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Pharmaceutical Regulatory Affairs Societies in USA: National Association of Pharmacy Regulatory Authorities, Regulatory Affairs Professionals Society (RAPS), Regulatory Affairs - American Chemical Society, The Organisation for Professionals in Regulatory Affairs; Regulatory Affairs North America, Society of Quality Assurance
Pharmaceutical Regulatory Affairs Societies in Europe: European Society of Regulatory Affairs, Legal and Regulatory Affairs Committee
Pharmaceutical Regulatory Affairs Societies in Asia and Pacific: Regulatory Affairs Professionals Society Singapore, Pharmaceutical Society of Australia, Pharmacy Guild of Australia, Chinese American Biopharmaceutical Society.
Pharmaceutical Regulatory Affairs Journals: Pharmaceutical Regulatory Affairs: Open Access, International Journal of Research and Development in Pharmacy & Life Sciences, International Journal of Drug Regulatory Affairs (IJDRA), Journal of Pharmaceutical Research and Regulatory Affairs and Pharmaceutical Drug Regulatory Affairs Journal (PDRAJ)
Track 21: Industrial and Physical Pharmacy
Industrial pharmacy is a discipline which includes manufacturing, development, marketing and distribution of drug products including quality assurance of these activities. The research topics are focused on solving current general problems in pharmaceutical industry conferences, such as formulation 2018 and characterization of sticky amorphous drugs, problem-solving for paediatric medicines and miniaturization of manufacturing processes.
Physical pharmacy conferences incorporate information of arithmetic, material science and science and apply them to the pharmaceutical dose frame improvement. Physical pharmacy gives the premise to understanding the synthetic and physical wonders that oversee the in vivo and in vitro activities of pharmaceutical items.
For more information visit: Pharmaceutical Conferences
Related Pharmaceutical Industry Conferences: Pharmaceutical Events | Pharmaceutical Conferences | Physical Pharmacy Conferences | Industrial Pharmacy Meetings | Physical Pharmacy Congress | Pharma Industry | Pharma Industry Experts | Pharmaceutical Industry Conferences | Pharma Marketing & Industry Conferences 2018 | Pharmaceutical Industry Meetings | Pharmaceutical Sciences 2019 | Pharmaceutics Conferences | Euro Pharmaceutics Conferences | Pharmaceutica 2019 | Euro Bio Pharmaceutics Conferences | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Pharmaceutical Industry Societies in USA: American College of Clinical Pharmacy (ACCP), American Pharmacy Association (APhA), American Society of Health-System Pharmacists (ASHP), National Association of Boards of Pharmacy (NABP), National Association of Specialty Pharmacy (NASP), National Community Pharmacists Association (NCPA), The Pharmaceutical Research and Manufacturers of America (PhRMA), The International Society for Pharmaceutical Engineering (ISPE), The American Association of Pharmaceutical Scientists (AAPS) and The Controlled Release Society (CRS)
Pharmaceutical Industry Societies in Europe: The European Federation of Pharmaceutical Industries and Associations (EFPIA), The European Generic Medicines Association (EGA), The European Personalised Medicine Association EPEMED, Association of the British Pharmaceutical Industry, International Federation of Pharmaceutical Manufacturers & Associations (IFPMA)
Pharmaceutical Industry Societies in Asia and Middle East: The Society of Hospital Pharmacists of Australia, Chinese Pharmaceutical Association, The Pharmaceutical Association of Israel, Kuwait Pharmaceutical Association and Pharmaceutical Society of New Zealand
Pharmaceutical Industry Journals: Journal of Developing Drugs, Future Journal of Pharmaceutical Sciences, Journal of Pharmaceutical Innovation, Advanced Drug Delivery Reviews, Drug Development and Industrial Pharmacy, European Journal of Pharmaceutical Sciences, European Journal of Pharmaceutics and Biopharmaceutics, International Journal of Pharmaceutics, Journal of Pharmacy and Pharmaceutical Sciences and The Pharmaceutical Journal
Track 22: Clinical and Hospital Pharmacy
Clinical pharmacy is a health science discipline in which pharmacists provide patient care that optimizes medication therapy and promotes health, and disease prevention. The practice of clinical pharmacy conferences embraces the philosophy of pharmaceutical care, blending a caring orientation with specialized therapeutic knowledge, experience, and judgment to ensure optimal patient outcomes. As a discipline, clinical pharmacy also has an obligation to contribute to the generation of new knowledge that advances health and quality of life.
Hospital pharmacy is a specialization of this field that includes additional duties such as aiding doctors in applying drug therapy. The statements were developed by the profession to bring uniformity to the practice of hospital pharmacy.
For more information visit: Pharmaceutical Conferences
Related Clinical Pharmacy Conferences: Clinical Pharmacy 2018 | Pharmaceutical Events | Pharmaceutical Conferences | Clinical Pharmacy Events | Clinical Pharmacy Meetings | Clinical Pharmacy Congress | Pharmaceutical Sciences Conferences | Pharmacology Meetings | Pharmaceutical Sciences Events | Pharmaceutical Sciences Exhibitions | Pharmaceutical Sciences Meetings | Pharma Industry Conferences | Pharma Research Conferences | | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Clinical Pharmacy Societies in USA: Academy of Managed Care Pharmacy, Accreditation Council for Pharmacy Education, American Association of Colleges of Pharmacy, American College of Clinical Pharmacy, American Pharmacists Association, American Society of Consultant Pharmacists, American Society of Health-System Pharmacists, National Alliance of State Pharmacy Associations, National Association of Boards of Pharmacy, National Community Pharmacists Association and Clinical Pharmacy Associates
Clinical Pharmacy Societies in Europe: The United Kingdom Clinical Pharmacy Association (UKCPA), Pharmacy Research UK, European Society of Clinical Pharmacy (ESCP), Association of Pharmacy Technicians UK, The Royal Pharmaceutical Society, Primary Care Pharmacy Association, Pharmaceutical Care Network Europe and European Society of Oncology Pharmacy - ESOP
Clinical Pharmacy Societies in Asia and Middle East: Pharmaceutical Society of Singapore, Pharmaceutical Society of Australia, The Pharmacy Guild of Australia, The Pharmaceutical Society of Hong Kong, Japan Pharmaceutical Association and The Korean Pharmaceutical Association.
Clinical Pharmacy Journals: International Journal of Clinical Pharmacy, International Journal of Research and Development in Pharmacy & Life Sciences, Journal of Clinical Pharmacy and Therapeutics, Journal of Hospital and Clinical Pharmacy, Journal of Basic and Clinical Pharmacy (JBCP), European Journal of Clinical Pharmacy and Korean Journal of Clinical Pharmacy (KJCP)
Track 23: Pharmacovigilance and Drug Safety
Pharmacovigilance is the science and exercises identifying with the discovery, evaluation, comprehension and counteractive action of unfavorable impacts or some other pharmaceutical related issue. Pharmacovigilance Conferences underpins general wellbeing programs by giving solid data to the productive evaluation of the hazard advantage profile of solutions, add to the appraisal of formal, uses, symptoms, damage, viability and danger of pharmaceuticals, empowering the sheltered, sound and more viable utilization of different medications. Advance instruction, understanding and clinical preparing in Pharmacovigilance and its successful accessibility to people in general.
Drug safety is otherwise called Medication Safety in the field of health. It is related with antagonistic impacts of Pharmaceutical items including numerous other logical perspectives, for example, the reactions of medications, the nature of solutions, prescription mistake in utilization of medications, absence of viability of medications, and fake medications. Tolerant Safety, Drug Interaction (drug–drug and food–drug cooperation) Drug Pharmacokinetic, and Adverse Drug Reaction are a few terms required with Drug Safety.
For more information visit: Pharmaceutical Conferences
Related Pharmacovigilance Conferences: Global Pharmacovigilance 2019 | Pharmacovigilance Conferences | Drug safety Conferences | Pharmacovigilance Events | Pharmacovigilance 2019 | Pharmacovigilance Meetings | Pharma Exhibitions | Pharma Symposiums | Pharma Marketing | Pharma Biotech Conferences | Top Pharma Conferences USA | Top Pharma Conferences Europe | Top Pharma Conferences Asia | Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Pharmacovigilance Societies in USA: International Society for Pharmacoepidemiology, Regulatory Affairs Professionals Society (RAPS), Medical Dictionary for Regulatory Affairs (MedDRA),
Pharmacovigilance Societies in Europe: Drug Safety Research Unit, The International Society of Pharmacovigilance (ISoP), Pharmaceutical Information & Pharmacovigilance Association, European Medicines Agency, Heads of Medicines Agency (HMA), Medicines and Healthcare Products Regulatory Agency (MHRA) and Irish Medicines Board (IMB) Committee for Medicinal Products for Human Use (CHMP), Pharmacovigilance Risk Assessment Committee (PRAC), Committee for Medicinal Products for Veterinary Use (CVMP), Committee for Orphan Medicinal Products (COMP), Committee on Herbal Medicinal Products (HMPC), Committee for Advanced Therapies (CAT), Health Products Regulatory Authority (HPRA)
Pharmacovigilance Societies in Asia & Middle East: International Conference on Harmonisation (ICH), Drug Information Association
Pharmacovigilance Journals: Journal of Pharmacovigilance, Symbiosis online Journal of Pharmacovigilance, Pharmacology and Pharmacovigilance Journal, International Journal of Pharmacovigilance and Journal of Pharmacovigilance and Pharmacotherapeutics
Track 24: Pharmacy Education and Practice
Standards of initial education and training for pharmacists set out the criteria against which we will approve education and training for student pharmacists and pre-registration trainee pharmacists. The standards ensure that newly registered pharmacists are competent to practice safely and effectively. The mission of pharmacy education is to prepare graduates who provide patient centered care that ensures optimal medication therapy outcomes and provides a foundation for specialization in specific areas of pharmacy practice; to participate in the education of patients, other health care providers and future pharmacists, to conduct research and scholarly activity and to provide service and leadership to the community.
For more information visit: Pharmaceutical Conferences
Related Pharmacy Education Conferences: Pharmacy Education Conference 2018 | Pharmacy Education 2019 | Pharmaceutical Events | Pharmaceutical Conferences | Pharma Conferences | Pharma Congress | Pharma Gatherings | Pharma Experts | Pharma Faculty | Pharma Companies | Pharma Seminars | Pharma Delegates | Pharma Speakers | Pharma University | Pharma researchers | Pharma Meetings | Pharma Networking | Pharma Societies | Pharma Middle East | World Pharma Conferences | Pharmacy Conferences
18th World Pharma Congress during October 18-20, 2018 Warsaw, Poland; 4th International Conference on Drug Discovery and Advanced Drug Delivery Systems conferences, October 18-19, 2018 at Warsaw, Poland; 18th Annual Pharmaceutical Chemical Analysis Congress, November 05-06, 2018 at Madrid, Spain; 9th Neuropharmacology Meeting during November 15-16, 2018 at Frankfurt, Germany; 3rd International Conference on Generics Drugs and Biosimilars Conferences, November 15-17, 2018 at Frankfurt, Germany; 23rd International Pharmaceutical Biotechnology Conference, December 10-11, 2018 at Rome, Italy; 19th Pharmaceutical Sciences and Pharma Industry Conference February 25-26, 2019, Berlin, Germany; 12th European Biosimilars Congress April 15-16, 2019 Berlin, Germany
Related Societies:
Pharmacy Education Societies in USA: Accreditation Council for Pharmacy Education, American Association of Colleges of Pharmacy (AACP), American Pharmacists Association (APhA), American Society for Pharmacy Law, American Society of Consultant Pharmacists (ASCP), American Society of Health-System Pharmacists (ASHP), Professional Compounding Centers of America, American College of Clinical Pharmacy (ACCP), College of Psychiatric and Neurologic Pharmacists (CPNP)
Pharmacy Education Societies in Europe: Association of the British Pharmaceutical Industry (ABPI), British National Formulary (BNF), British National Formulary for Children (BNFC), British Pharmacopoeia Commission (BPC), Commission on Human Medicines, General Pharmaceutical Council (GPhC), Medicines and Healthcare products Regulatory Agency (MHRA), National Institute for Health and Clinical Excellence (NICE), National Patient Safety Agency (NPSA), Pharmaceutical Services Negotiating Committee (PSNC), Royal Pharmaceutical Society (RPS), Scottish Medicines Consortium (SMC), UK Clinical Pharmacy Association, UKMedix (UKM), Worshipful Society of Apothecaries of London and General Pharmaceutical Council
Pharmacy Education Societies in Asia and Pacific: National Alliance for Pharmacy Education, Pharmacy Council of India, Pharmaceutical Society of New Zealand, Pakistan Pharmacists Association, Pharmaceutical Association of Mauritius, Kuwait Pharmaceutical Association, The Pharmaceutical Association of Israel, Chinese Pharmaceutical Association, The Society of Hospital Pharmacists of Australia, The Pharmacy Guild of Australia, Pharmaceutical Society of Australia and Australian College of Pharmacy
Related Pharmacy Education Journals: American Journal of Pharmaceutical Education, Pharmacy, Saudi Pharmaceutical Journal, Currents in Pharmacy Teaching and Learning, Archives of Pharmacy Practice, Journal of Advanced Pharmacy Education & Research [JAPER], Journal of Pharmaceutical Care & Health Systems and Indian Journal of Pharmacy Practice

Welcome Message
by
Dr. John D. Higgins
Senior Director, Discovery Pharmaceutical Sciences
MSD, USA
On behalf of the Organizing Committee and as its Chair, it’s my pleasure to welcome participants to the 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems - “Pharmaceutica 2019”, in Edinburgh, Scotland, during March 19-20 2019. The theme is “Shaping the future of Pharmaceutical Industry and Drug delivery systems”
This year’s conference will be held during March 19-20 2019 in Edinburgh, Scotland and is the continuation of a highly successful series of global meetings featuring novel drug delivery strategies. The series is organized by Conference Series llc LTD, a leading organization that specializes in pharmaceutical conferences throughout the globe. I’ve had the pleasure of lecturing at past Pharmaceutica conferences in Berlin and San Diego and I’m looking forward to yet another edition of this excellent conference series.
The development of novel and effective methods to deliver drugs specifically to their molecular target is essential for new and established drugs alike. Furthermore, preclinical and clinical development of any new drug should go hand-in-hand with exploring new approaches towards its delivery at the tissue, cellular and subcellular levels. Pharmaceutica 2019 promises to deliver on these interesting broad topics.
We expect Pharmaceutica 2019 to attract scientists and decision-makers from both academia and industry, with internationally renowned Keynote and Plenary Speakers who will introduce and discuss cutting edge research in diverse areas such as pre-formulation, delivery routes and drug delivery technologies. The venue also will include discussions on major drug delivery challenges, relevant physiological considerations, drug targeting, diverse applications of nanotechnology and drug delivery systems for vaccines and biologics. In addition, interesting adjacent areas such as medical devices as well as aspects of global drug discovery/development policy also will be covered. As with any high-quality conference venue, Pharmaceutica 2019 also will include a large number of industrial exhibitors, and a variety of innovative educational sessions and workshops.
I’m confident that all participants, students, experts and policy makers alike will greatly benefit from attending Pharmaceutica 2019 and I’m looking forward to an interesting and informative meeting with stimulating discussions.
Last but not least, I’m excited to meet many new colleagues working in drug delivery and related areas.
John D. Higgins
Senior Director and Global Technology Lead, Discovery Pharmaceutical Sciences;
MSD, USA
After a successful conference of Pharmaceutica 2018, Pharmaceutical Conferences team cordially invites participants from all over the world to attend "20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems" (Pharmaceutica 2019) slated on March 18-20, 2019 at Edinburgh, Scotland. This Pharmaceutics Conference includes a wide range of Keynote presentations, Oral talks, Poster presentations, Symposia, Workshops, Exhibitions and Career development programs.
Novel drug delivery systems Conference is one of the well-established conference among Pharmaceutical Conferences organized by Conference Series llc Ltd
Pharmaceutica 2019 covers various aspects of Bio-Pharmaceutics, Pharmaceutical Chemistry, Drug Targeting and Design, Pharmacokinetics and Pharmacodynamics in Drugs, Pharmaceutical Formulation, Pharmaceutical Manufacturing, Pharmaceutical Nanotechnology, Novel Drug Delivery Systems, Smart Drug Delivery Systems, Nanomedicine and Biomedical Applications, Biomaterials in Drug Delivery, Vaccine Drug Delivery Systems, Medical Devices for Drug Delivery, Biologics & Biosimilars, Pharmaceutical Analysis, Pharmaceutical Process Validation, Pharmaceutical Packaging, Clinical Trials and Clinical Research, Pharmacogenetics and Genomics, Regulatory Affairs and Intellectual Property Rights, Industrial and Physical Pharmacy, Clinical and Hospital Pharmacy, Pharmacovigilance and Drug Safety, Pharmacy Education and Practice
2019 Highlights:
- 200+ Participation (70% Industry: 30% Academia)
- 9+ Keynote Speakers
- 30+ Plenary Speakers
- 3+ Exhibitors
- 14 Innovative Educational Sessions
- B2B Meetings
Why you should attend?
- Access to senior-level executives from leading international pharmaceutical & biotechnology companies
- Exposure to new and exciting companies ready for partnerships and licensing opportunities in the U.S., Europe and Asia
- Tremendous networking opportunities to meet numerous U.S., European, Japanese and Asian biotech and pharmaceutical companies in one place at one time
- Use of state-of-the-art partnering technology
- Targeted partner selections through proprietary screening process
- Get to the heart of why formulation and delivery strategies fail. Dissect the challenges before looking for concrete solutions.
- Discover how advances in the sector are impacting both large and small molecule drugs.
- Explore tried and tested routes to improve bioavailability.
- Understand how to develop the right formulation and delivery strategy with a strong scientific, clinical and commercial mind set.
- Discover the latest innovations in drug delivery devices.
- Be inspired by innovative case studies and realize the potential impact on your formulation or delivery processes.
- Engage in the exciting event format, with round tables, panels, showcases, speed networking and multiple conference tracks.
- Share experiences, insights and strategies in interactive peer to peer round tables.
- Hear more perspectives in one place – from large medium and small organizations from pharma, biotech and academia.
- Discover how scientific formulation advancements are being implemented in practice.
Who Should Attend
- Directors, CEO’s of Organizations
- Business Development Managers
- Chief Scientific Officers
- R&D Researchers from Biosimilar and Biologics Industries
- Professors, Associate Professors, Assistant Professors
- PhD Scholars
- Patent Attorneys
- Intellectual Property Attorneys
- Investment Analysts
- Association, Association presidents and professionals
- Drug development and discovery companies
- Formulation and Pharma Manufacturing companies
- Medical technology companies
- Universities and institutes
- Intellectual property and legal organizations
- Investors and financial services providers
- Bio-clusters and incubators
- Government and public support agencies
- Cosmetic companies
- Generic drugs and Biosimilars
Pharmaceutics & Drug Delivery Market Research:
Pharmaceuticals are one of the world's most profitable industries. During the last 30 years, the industry has spent billions of dollars on research and reaped billions in return. In 2008 alone, the pharmaceutical industry sold $773 billion in products worldwide-a number that has consistently grown for the past 8 years and is projected to increase again by 2.5 to 3.5 percent in 2017.
*Source: IMS Health
The Market report of most of the pharmaceutical companies shows their R&D investments majorly in the following areas, namely:
- Advanced Drug Delivery Systems
- Antibiotics
- Excipients in Pharmaceuticals
- Ophthalmic Therapeutic Drugs
- Skin Disease Treatment
- Vaccine Technologies
- Breakthrough Therapies
Advanced Drug Delivery Systems:
- The global advanced drug delivery market should grow from roughly $178.8 billion in 2015 to nearly $227.3 billion by 2020, with a compound annual growth rate (CAGR) of 4.9%.
- The North American market should grow from nearly $75.7 billion in 2015 to $93.4 billion by 2020, a CAGR of 4.3%.
- The European market should grow from roughly $57.3 billion in 2015 to nearly $72.1 billion by 2020, a CAGR of 4.7%.
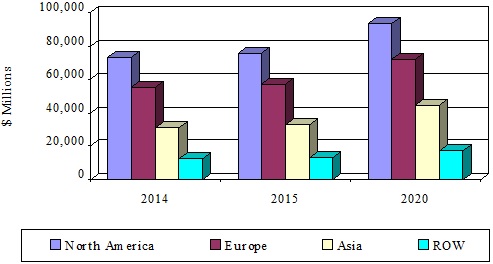
*Source: BCC Research
Antibiotics:
- The global systemic antibiotics market should reach nearly $44.7 billion in 2020 from nearly $40.6 billion in 2015 at a compound annual growth rate (CAGR) of 2.0% from 2015 to 2020.
- The beta-lactams market should reach over $22.0 billion by 2020 from over $20.6 billion in 2015, a CAGR of 1.3% from 2015 to 2020.
- The other antibiotic classes market should reach over $10.6 billion in 2020 from nearly $8.3 billion in 2015, a CAGR of 5.0% from 2015 to 2002.
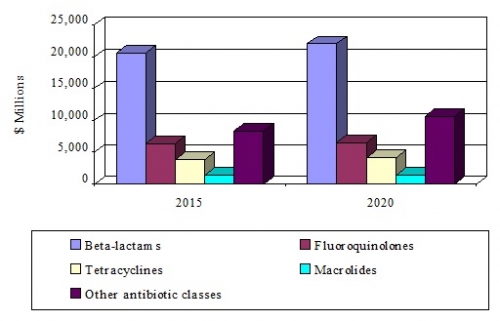
*Source: BCC Research
Excipients in Pharmaceuticals:
- The global excipients market should reach nearly $6.9 billion by 2020 from over $6.2 billion in 2015, a compound annual growth rate (CAGR) of 1.9% from 2015 to 2020.
- The organic excipients market should reach $6.3 billion by 2020 from nearly $5.8 billion in 2015, a CAGR of 1.7% from 2015 to 2020.
- The inorganic excipients market should reach $433.7 million by 2020 from $351.9 million in 2015, a CAGR of 4.3% from 2015 to 2020.
*Source: BCC Research
Ophthalmic Therapeutic Drugs:
- The global ophthalmic therapeutic drug market was valued at $12.3 billion in 2014. This market is expected to reach $19 billion by 2019, with a compound annual growth rate (CAGR) of 9.1% from 2014 to 2019.
- The global age-related macular degeneration (AMD) market is expected to grow to nearly $7.9 billion by 2019 from nearly $5.4 billion in 2014, a CAGR of 7.8% from 2014 to 2019.
- The global glaucoma therapeutic market generated revenue of $3.8 billion in 2014, and by 2019 this segment is expected to generate $6.4 billion, with a CAGR of 11.1% from 2014 to 2019.
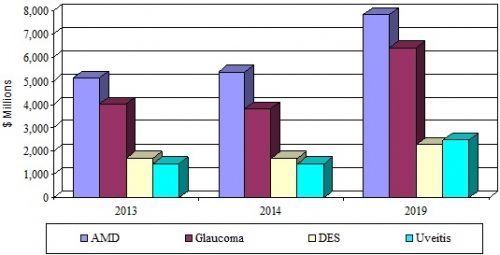
*Source: BCC Research
Skin Disease Treatment:
- The global market for skin disease treatment technologies reached $16.4 billion in 2014 and $17.1 billion in 2015. The market should reach $20.4 billion in 2020, demonstrating a compound annual growth rate (CAGR) of 3.6% from 2015 to 2020.
- The U.S market for skin disease treatment dominates the global market throughout the period, and totaled $7.3 billion in 2014. The market should reach $7.5 billion in 2015 and $8.6 billion in 2020 at a CAGR of 2.6% from 2015 to 2020.
- BRIC (Brazil, Russia, India, and China) is the fastest growing region of the global dermatology market with a CAGR of 6% from 2015 to 2020. The market totaled $3.5 billion in 2015 and should reach more than $4.6 billion by 2020.
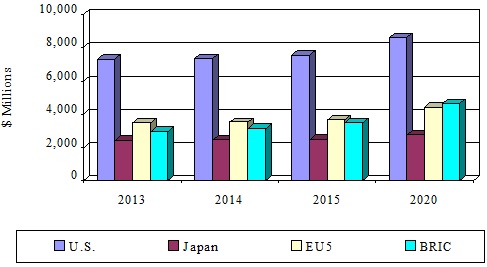
*Source: BCC Research
Vaccine Technologies:
- The global market for vaccine technologies reached $33.3 billion in 2016 and should reach $45.2 billion by 2021, growing at a compound annual growth rate (CAGR) of 6.3% from 2016 to 2021.
- Human vaccines as a segment of this market reached $27.2 billion in 2016 and should reach nearly $37.5 billion by 2021, growing at a CAGR of 6.6% from 2016 to 2021.
- Animal vaccines as a segment of this market reached nearly $6.1 billion in 2016 and should reach $7.7 billion by 2021, growing at a CAGR of 4.9% from 2016 to 2021.
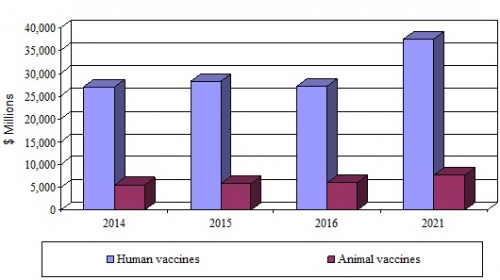
*Source: BCC Research
Breakthrough Therapies:
- The global market for breakthrough therapy designation (BTD) drugs should reach $99.2 billion by 2022 from $48.8 billion in 2017 at a compound annual growth rate (CAGR) of 15.2%, from 2017 to 2022.
- The cancer therapy segment of the breakthrough therapy designation drugs industry is the largest market. The market is expected to grow from $19.6 billion in 2017 to $58.6 billion in 2022 at a CAGR of 24.5% for the period 2017-2022.
- The CNS and neurology therapy segment of the breakthrough therapy designation drugs industry is expected to grow from $956 million in 2017 to $8.4 billion in 2022 at a CAGR of 54.3% for the period 2017-2022.
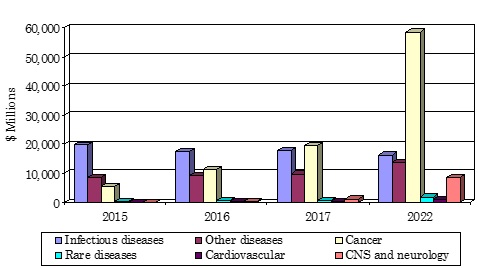
*Source: BCC Research
2017 Pharmaceuticals Research Review:
Pharmaceuticals represented a US$300 bn-a-year market globally as of 2015, the World Health Organization states. The global pharmaceutical market is expected to surpass US$400 bn by 2018, with the ten largest pharmaceutical companies collectively commanding about a third of the market. Companies in the pharmaceutical industry are characterized by their sizeable expenditure on R&D and marketing initiatives in a bid to rake in more revenue. The development of biopharmaceuticals represents a milestone for the industry and personalized therapies carry immense promise in the near future.
Highlights:
- The global market for cancer vaccines totaled $4.5 billion in 2013 and reached nearly $4.0 billion in 2014. This market is expected to reach $4.3 billion by 2019, registering a compound annual growth rate (CAGR) of 1.3% for the period 2014-2019.
- The global market for “silent” cancers was valued at $8.5 billion in 2013 and $9 billion in 2014. This market is expected to reach almost $13.6 billion by 2019, with a CAGR of 8.5% from 2014 to 2019.
- The global market for incretin-based therapeutics was valued at nearly $11.8 billion in 2013 and $12.7 billion in 2014. This market is expected to reach $22.8 billion by 2019, with a CAGR of 12.4% from 2014 to 2019.
Cancer Vaccines: Technologies and Global Markets:
The global human vaccines market was valued at US$28.3 bn in 2015 and is estimated to reach US$72.5 bn by 2024, registering an 11.2% CAGR during the forecast period. Extensive promotion and marketing strategies and favourable government support are likely to boost market growth.
The global market for cancer vaccines was valued at nearly $4.2 billion and $4.0 billion in 2014 and 2015, respectively. This market is expected to decline from nearly $3.5 billion in 2016 to $3.4 billion in 2021 at a compound annual growth rate (CAGR) of -0.4% for 2016-2021.
Drug Delivery Companies:
The global drug delivery technology market is projected to reach USD 1,669.40 Billion by 2021 from USD 1,179.20 Billion in 2016, at a CAGR of 7.2% during the forecast period. This market is segmented based on route of administration, facility of use, and region.
The drug delivery technology is highly competitive market, comprising of various players. Prominent players in the drug delivery technology market include Johnson & Johnson, Inc. (U.S.), F. Hoffman-La Roche (Switzerland), Merck & Co., Inc. (U.S.), Bayer AG (Germany), Pfizer, Inc. (U.S.), Novartis AG (Switzerland), 3M Company (U.S.), Becton, Dickinson and Company (U.S.), GlaxoSmithKline plc, (U.K.), Sanofi (France), and Antares Pharma, Inc. (U.S.).
Nanotechnology Drug Delivery Market:
The global nanotechnology drug delivery market was valued at US$ 41,062.5 Mn in 2014 and is projected to reach US$ 118,527.2 Mn by 2023, expanding at a CAGR of 12.5% from 2015 to 2023.
Key players having presence in the global nanotechnology drug delivery market are AbbVie, Inc., Amgen, Inc., Celgene Corporation, Johnson & Johnson, Merck & Co., Inc., and Novartis International AG, among others.
Active Pharmaceutical Ingredient Market:
Transparency Market research states the opportunity in this market will be worth US$219.60 bn by 2023 from US$134.7 bn in 2015. Between the forecast period of 2015 and 2023, the overall market is expected to expand at a CAGR of 6.3%.
Some of the leading companies operating in the global active pharmaceutical ingredient market are Zhejiang Medicine Co., Ltd., Teva Pharmaceutical Industries Ltd., Zhejiang NHU Co., Ltd , North China Pharmaceutical Group Corp. (NCPC), Dr.Reddy’s Laboratories Limited, Northeast Pharmaceutical Group Co., Ltd., Aurobindo Pharma Limited, Sandoz (Novartis AG), Zhejiang Hisun Pharmaceutical Co., Ltd. and Zhejiang Huahai Pharmaceuticals Co., Ltd. amongst others. Currently, these players are focusing on partnering with other companies to acquire a new line of products to add value to their portfolio. Furthermore, companies are also anticipated to focus on expanding their capacities to cater to the vast unmet medical needs of the world.
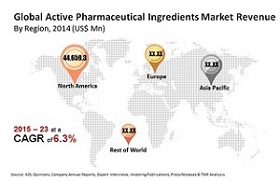
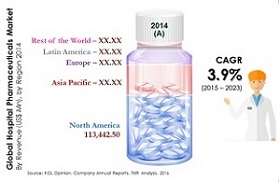
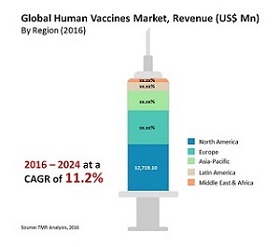
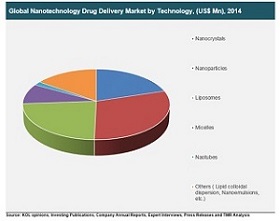
Target Audience:
- Drug Delivery Technology Manufacturers
- Public and Private Physicians
- Healthcare Institutions (Medical Data Centers)
- Research & Clinical Laboratories
- Distributors and Suppliers of Drug Delivery Technologies
- Health Insurance Payers
- Market Research and Consulting Firms
Conference Highlights
- Pharmaceutical Chemistry
- Pharmacokinetics and Pharmacodynamics in Drugs
- Drug Targeting and Design
- Pharmaceutical Formulation
- Pharmaceutical Manufacturing
- Pharmaceutical Nanotechnology
- Novel Drug Delivery Systems
- Smart Drug Delivery Systems
- Nanomedicine and Biomedical Applications
- Biomaterials in Drug Delivery
- Vaccine Drug Delivery Systems
- Medical Devices for Drug Delivery
- Pharmaceutical Analysis
- Biologics & Biosimilars
- Bio-Pharmaceutics
- Pharmaceutical Packaging
- Pharmaceutical Process Validation
- Clinical Trials and Clinical Research
- Pharmacogenetics and Genomics
- Regulatory Affairs and Intellectual Property Rights
- Industrial and Physical Pharmacy
- Clinical and Hospital Pharmacy
- Pharmacovigilance and Drug Safety
- Pharmacy Education and Practice
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | March 18-20, 2019 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Pharmaceutics & Drug Delivery Research
- Journal of Nanomedicine & Nanotechnology: Open Access
- Journal of Pharmaceutical Sciences & Emerging Drugs
Abstracts will be provided with Digital Object Identifier by