Amélia C F Vieira
University of Coimbra, Portugal
Title: Diclofenac-β-cyclodextrin for colonic drug targeting: From synthesis to in vivo performance in rats
Biography
Biography: Amélia C F Vieira
Abstract
A new conjugate of diclofenac with β-cyclodextrin through an ester linkage has been synthesized. Diclofenac is a non-steroidal anti-inflammatory drug, its targeting to the colon would provide a suitable tactic for the management of arthritis pain by chronotherapy with simultaneous circumvention of the adverse gastric effects associated with the free drug. β-cyclodextrin is a cyclic oligosaccharide that can function as a carrier to develop colon-targeted prodrugs through the synthesis of a suitable covalent linkage with a drug. However, it was required to exploit various strategies to form the required ester linkage between the diclofenac and β-cyclodextrin. Only the nucleophile substitution of mono-6-tosyl-β-cyclodextrin under microwave irradiation allowed an efficient successful synthesis. The conjugate was identified by proton nuclear magnetic resonance (1H-NMR) spectroscopy and matrix-assisted laser desorption/ionization (MALDI) spectra. Stability of the diclofenac-β-cyclodextrin conjugate were carried out in human fecal slurries and in simulated gastric and intestinal fluids. Results demonstrated that the conjugate released diclofenac at the level of the lower intestine, and exhibited good stability in the upper gastrointestinal tract. As a proof-of-concept, a comparative in vivo study in fasted rats was performed by oral administration of a suspension of prodrugs, diclofenac-β-cyclodextrin and a well-known prodrug, sulfasalazine to a group of rats. A lag time between oral intake of prodrugs and the appearance of the respective drugs in plasma was observed. Overall, this study confirms the in vivo ability of our newly cyclodextrin prodrug to target and release diclofenac specifically in the colon.
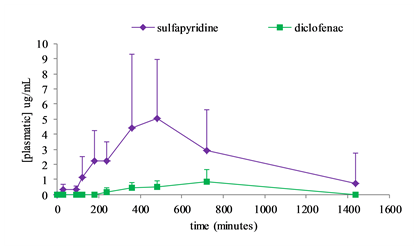
Figure : Concentration-time profiles of diclofenac and sulfapyridine in Wistar rats after simultaneous oral administration of diclofenac-β-cyclodextrin (88.5 mg/kg) and sulfasalazine (100 mg/kg). Each bar represents mean±SD (n = 7).