Day 1 :
Keynote Forum
Vladimir P Torchilin
Northeastern University, USA
Keynote: Stimuli-sensitive nanopreparations for multidrug resistant cancer
Time : 09:00-09:35

Biography:
Vladimir P Torchilin, PhD, DSc, is a University Distinguished Professor of Pharmaceutical Sciences and Director at Center for Pharmaceutical Biotechnology and Nanomedicine, Northeastern University, Boston. His interests include drug delivery and targeting, nanomedicine, multifunctional and stimuli-sensitive pharmaceutical nanocarriers, biomedical polymers and experimental cancer therapy. He has published more than 400 original papers, more than 150 reviews and book chapters, wrote and edited 12 books, and holds more than 40 patents. Google Scholar shows more than 50,000 citations of his papers with H-index of 100. He is Editor-in-Chief of Current Drug Discovery Technologies, Drug Delivery, and OpenNano, Co-Editor of Current Pharmaceutical Biotechnology and on the Editorial Board of many other journals. He received more than $30 M from the governmental and industrial sources in research funding. He has received multiple honors and awards and in 2011, Times Higher Education ranked him number two among Top World Scientists in Pharmacology for the period of 2000-2010
Abstract:
Tumor therapy, especially in the case of multidrug resistant cancers, could be significantly enhanced by down-regulating the production of proteins, which are involved in cancer cell resistance, such as Pgp or survivin. This can be achieved by using corresponding siRNA. Even better response could be achieved if such siRNA could be delivered to tumors together with chemotherapeutic agent. This task is complicated by low stability of siRNA in biological surrounding. Thus, the delivery system should simultaneously protect siRNA from degradation. We have developed several types of lipid-core polymeric micelles based on PEG-phospholipid or PEI-phospholipid conjugates, which are biologically inert, demonstrate prolonged circulation in the blood and can firmly bind non-modified or reversibly-modified siRNA. Additionally, these nanopreparations can be loaded into their hydrophobic compartments with poorly water soluble chemotherapeutic agents, such as paclitaxel or camptothecin. In experiments with cancer cell monolayers, cancer cell 3D spheroids, and in animals with implanted tumors, it was shown that such co-loaded preparations can significantly down-regulate target proteins in cancer cells, enhance drug activity, and reverse multidrug resistance. In order to specifically unload such nanopreparations inside tumors, we made them sensitive to local tumor-specific stimuli, such as lowered pH, hypoxia, or overexpressed certain enzymes, such as matrix metalloproteases. Using pH-, hypoxia-, or MMP2-sensitive bonds between different components of nanopreparations co-loaded with siRNA and drugs, we were able to make the systems specifically delivering biologically active agents in tumors, which resulted in significantly improved therapeutic response. We have also developed approaches to target individual intracellular organelles to initiate the apoptosis in resistant cancer cells.
Keynote Forum
Alain L Fymat
International Institute of Medicine and Science, USA
Keynote: Therapeutics delivery behind, through and beyond the blood brain barrier
Time : 09:35-10:10

Biography:
Alain L Fymat is a Medical-Physical Scientist who completed his education at the Universities of Bordeaux and Paris-Sorbonne, France, and the University of California at Los Angeles. He is the current President/CEO and Professor at the International Institute of Medicine and Science. He was formerly Professor of Radiology, Radiological Sciences, Radiation Medicine (Oncology), Critical Care Medicine, and Physics at several US and European universities. His current research interests lie at the interface between science and medicine (Precision Medicine, Nanobiotechnology, Nanomedicine, Genetics/Epigenetics/Ecogenetics), and drug delivery across the blood brain barrier. He has extensively published (~350 scholarly publications) and lectured in several national and international academic, professional, governmental and industrial venues. He is a Board Member of several institutions, Honorable Editor of International Journals of Journal of Cancer Prevention & Current Research, Nanomedicine Research Journal, Journal of Nanomedicine and Nanotechnology, Open Access Journal of Surgery, Nanomedicine, Journal of Nanobiotechnology, Journal of Tumor Medicine & Prevention, Nanomedicine & Nanoscience Research, Cancer Therapy and Oncology, Cancer Research and Clinical Oncology and Biomedical Journal of Scientific and Technical Research
Abstract:
There are approximately 400 known neural disorders some of which being due to a disruption or failure (breakdown, opening, damage) of the blood brain barrier (BBB), while other disorders may still have unknown effects on it. Examples include: meningitis (an inflammation of the meninges or membranes surrounding the brain and spinal cord); epilepsy (chronic or acute seizures caused by inflammation); multiple sclerosis (MS — a disease of the immune system or/and the breaking down of the BBB in a section of the brain or spinal cord); Alzheimer disease (AD — a disease in which amyloid beta contained in blood plasma enter the brain and adhere to the surface of astrocytes); possibly prion and prion-like diseases such as Parkinson disease (PD) and AD; HIV encephalitis (a precursor of HIV-associated dementia in which latent HIV can cross the BBB inside circulating monocytes in the blood stream); and systemic inflammation (sterile or infectious) that may lead to effects on the brain, cause sickness behavior and induce or/and accelerate brain diseases such as MS and PD. There are currently active investigations into treatments for a compromised BBB. As a consequence of the growing aging population, many such neurodegenerative diseases, cancer and infections of the brain will become more prevalent. Here are those disorders requiring treatment by delivery of drugs across, behind and beyond the BBB. It is therefore of utmost importance to grasp the difficulties encountered when attempting to deliver therapeutics at the right brain locations and at the right time-dose fractionations. This presentation will first briefly review the brain diseases and their effects on the BBB, discuss the early discovery and physiological development of the BBB, provide a brief primer on nanoneurology, and discuss the clinical significance of the BBB as a drug target. The drug delivery difficulties and their limitations, the drug targeting mechanisms (“behind”, “through” and “beyond” the BBB), and describe the novel uses offered by nanotechnology (nanoparticles and nano delivery devices) will then be discussed, followed by suggestions regarding approaches for the enhancement of drug delivery including physiological approaches, chemical and biological delivery, disruption of the BBB system, use of molecular Trojan horse systems. This will be concluded with future prospects in this research field.
Keynote Forum
Wei-Chiang Shen
University of Southern California, USA
Keynote: Human growth hormone–single-chain Fc-dimer fusion protein
Time : 10:10-10:45

Biography:
Wei-Chiang Shen is currently John A Biles Professor in Pharmaceutical Sciences at the University of Southern California, School of Pharmacy. His research interests focus on the development of novel systems for improving peptide and protein drug delivery. He has published 150 papers in different areas of biomedical sciences. He is a Co-author of the textbook, “Immunology for Pharmacy Students”, and a Co-editor of the book, “Antibody-Drug Conjugates – the 21st Century Magic Bullets for Cancer”. He was elected as Fellow of the American Association of Pharmaceutical Scientists (AAPS) in 1992 and Fellow of the American Association for the Advancement of Sciences (AAAS) in 2000, and was the recipient of the Grand Prize of Eurand Award for Outstanding Novel Research in Oral Drug Delivery from Controlled Release Society in 2002.
Abstract:
During the past decades, many Fc fusion proteins have been successfully produced by biotech companies and approved by FDA to be used as long-acting forms of protein drug. However, there are several limitations on the application of Fc-fusion protein technology to many proteins, especially the stability of Fc-dimerization. In this report, we describe a novel Fc-based drug carrier, single chain Fc-dimer (sc(Fc)2), which was designed to contain two Fc domains recombinantly linked together via a flexible linker. Since the Fc dimeric structure is maintained through the flexible linker, the hinge region was omitted to further stabilize it against proteolysis and to reduce Fc R-related effector functions. Our results indicate that the recombinant sc(Fc)2 preserved the neonatal Fc receptor (FcRn) binding capacity. We have prepared a fusion protein consisting human growth hormone (hGH) and sc(Fc)2 as a model of the novel Fc fusion protein. Compared to the monomeric hGH-Fc fusion protein, hGH-sc(Fc)2 fusion protein showed a stronger binding affinity to FcRn, a longer in vivo plasma half-life of hGH, and a higher hGH-induced plasma IGF-1 level in mice. Our results suggest that sc(Fc)2 technology has the potential to greatly advance and expand the use of Fc-technology for improving the pharmacokinetics and bioactivity of protein therapeutics
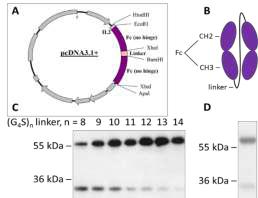
Figure : Production of single-chain Fc-dimer, sc(Fc)2, in HEK 293 cells. (A) Plasmid of sc(Fc)2 expression, (B) Structure of sc(Fc)2, (C) Anti-Fc WB (non-reducing conditions) of sc(Fc)2 with indicated (G4S) linker lengths. (D) Coomassie Blue gel (reducing conditions) of sc(Fc)_(G4S)13.
- Nanomedicine and Nanotechnology | Drug Delivery Technologies | Smart Drug Delivery Systems | 2D & 3D Printing in Drug Delivery | Pre-Formulation & Formulation Aspects | Drug Targeting and Design | Pharmaceutical Nanotechnology
Location: Zieten

Chair
Vladimir P Torchilin
Northeastern University, USA

Co-Chair
Wei-Chiang Shen
University of Southern California, USA
Session Introduction
Mikhail Durymanov
South Dakota State University, USA
Title: Nanoparticles for hepatoprotection against anticancer drugs
Time : 11:10-11:35

Biography:
Mikhail Durymanov has completed his PhD from Moscow State University and Postdoctoral Studies from Institute of Gene Biology. He joined Dr. Reineke’s group at South Dakota State University in 2016. His current research interests include cancer biology and immunology studies, development of 3D tumor models and nanomedicines for treatment of cancer and inflammatory diseases. He has published eight peer-reviewed papers.
Abstract:
During the last 50 years, cytotoxic chemotherapy has remained the major strategy against cancer. At the same time, hepatotoxicity of anticancer drugs is one of the major concerns associated with chemotherapy. Current liver chemoprotective agents are designed for systemic non-targeted applications. Besides liver, these medications also accumulate within the tumor and may potentially exert their chemoprotective effect on tumor tissues to the detriment of chemotherapeutic efficacy. In this study, we developed a new strategy for targeted delivery of protective drugs to the liver enabling higher anticancer drug doses without detrimentally impacting efficacy. Chemoprotective drug sulforaphane (SFN), which upregulates phase 2 and antioxidant defense enzymes, was encapsulated with loading efficiency of 14% (w/w) into biodegradable nanoparticles made of porous materials known as “metal-organic frameworks” (MOFs). After 24 h of incubation, SFN-MOF nanoparticles caused 2-fold increase of glutathione-S-transferase (GST) activity and enhanced expression of this phase 2 enzyme in mouse hepatocytes AML12. Moreover, SFN-MOF nanoparticles did not show any cytotoxicity and prevented caspase activation along with hepatocyte death after exposure to DOX. Treatment of tumor bearing mice with SFN-MOF nanoparticles led to significant GST upregulation in the liver, but not in the tumor; whereas use of free SFN enhances its expression in both tissues. Moreover, application of SFN-MOF nanoparticles prevented DOX-induced liver toxicity, and did not reduce therapeutic outcome. Thus, SFN-MOF nanoparticles provided preventive protection of the liver during chemotherapy and did not increase resistance of tumors to anticancer drug.
Fiorenza Rancan
Charité – Universitätsmedizin Berlin, Germany
Title: Drug delivery to skin by nanotechnology-based drug delivery systems
Time : 11:35-12:00

Biography:
Fiorenza Rancan is an Associated Scientist at the Clinical Research Center for Hair and Skin Science at the Charité University of Berlin Charité – Universitätsmedizin Berlin, Germany. Her expertise lays in dermal and transdermal drug delivery. She is interested in the interactions between nanocarriers and skin barrier components as well as skin immune cells. Her main research fields are dermatotherapy, transcutaneous vaccination, and chronic wounds with biofilm infections. She investigated several biodegradable particles (e.g. poly-lactic acid and virus-like particles) for transcutaneous vaccine delivery, explored the use of stimuli-responsive nanogels for the treatment of skin inflammatory conditions as well as delivery systems for the treatment of wound infections. She works on ex vivo human skin and skin organ culture to develop models for healthy and inflammatory skin as well as wound infections.
Abstract:
To overcome the sophisticated cutaneous barrier is one of the main issues of drug delivery to the skin. The stratum corneum can block microorganisms, particulate materials, and bulky molecules. In skin inflammatory diseases, where the stratum corneum is impaired, penetration of drugs is hindered by the component of the viable skin layers such as the tight junctions in the stratum granulosum. Even if the epidermis is missing, like in case of wounds, collage bundles in the dermis delay the penetration and diffusion of drugs. Nanotechnology-based delivery systems like thermoresponsive polyglycerol-based nanogels (tNGs) or nanowires have been shown to be valuable tools for selective and sustained release of drugs to skin. Recently, we used tNG to deliver tacrolimus (TAC), a high molecular weight poorly penetrating skin drug. We compared the particle-based formulation with the commercial formulation (Protopic 0.1%) using breast and abdominal ex vivo skin. Different methods for skin barrier disruption were used in order to investigate tNG skin penetration and drug release in skin with compromised barrier. The amount of penetrated TAC was measured in skin extracts by liquid chromatography–mass spectroscopy/mass spectroscopy (LC–MS/MS), whereas effects on skin inflammatory mediators (IL-6 and IL-8) were detected by means of ELISA. In another study we used polylactic-co-glycolic acid (PLGA) particles, as well as PVP-based nanofibers to deliver the antimicrobial drug ciprofloxacin to a wound model based on ex vivo human skin. The results showed that nanocarriers help to deliver drugs across the stratum corneum to the target skin regions. Different drug delivery profiles could be achieved depending on the delivery system. We conclude that nanotechnology offers promising alternatives to conventional drug formulations.
Sheng Qi
University of East Anglia, UK
Title: ‘Personalised pills’ by FDM 3D printing: Challenges and potential
Time : 12:00-12:25

Biography:
Sheng Qi is a Reader in Pharmaceutics at the School of Pharmacy, University of East Anglia. She has worked closely with pharmaceutical and excipient companies in the areas of pharmaceutical processing, physicochemical characterisation, and formulation development. Her current interests mainly focus on innovations in processing and material characterisation and engineering research which have applications in product development of the food, cosmetic and pharmaceutical industries.
Abstract:
Oral medicines manufactured using conventional tabletting and capsule filling methods can fulfil basic therapeutic functions as they are the most preferred route of administration due to their non-invasive and convenient nature. However, the adherence of the patients with chronic conditions such as cardiovascular disease, cancer and neuro-degenerative diseases have low adherence to prescribed medicines and with complex medicine regimens, adherence can be as low as 50%. This is a global problem and the lack of adherence to prescribed medicines is estimated to cost the NHS over £500 million each year. Clinical evidence has shown that using polypills (incorporating multiple medications into one pill) can significantly improve patient adherence to the treatment. 3D printing (3DP) can provide the high flexibility required for providing built-in sophisticated microstructures to host different drugs with easily adjustable doses in different parts of a single polypill and has well-recognised potential for personalised polypill manufactuering. However, there is a wide range of challenges that 3D printed personalized polypills face which are barriers for translation of the technology into clinical products. This presentation will discuss these challenges in detail and present the latest developments in technological strategies on how to overcome these issues in order to fast-track realization of the clinical potential of 3D printed personalized polypills.
N.Basaran Mutlu Agardan
Gazi University, Turkey
Title: Redox potential sensitive smart nanopreparations for drug and siRNA delivery in cancer
Time : 12:25-12:50

Biography:
N Basaran Mutlu Agardan graduated from Gazi University Faculty of Pharmacy, and obtained there her PhD from the Department of Pharmaceutical Technology. She then gained a scholarship from the Scientific and Technological Research Council of Turkey and completed her Post-doctoral research studies at the Center for Pharmaceutical Biotechnology and Nanomedicine, Northeastern University, Boston. Her area of research focuses on the smart drug delivery systems, liposomes, drug/gene delivery in cancer and oral absorption enhancement.
Abstract:
Cancer drug nanotechnology is a multidisciplinary area of research, aiming the delivery of therapeutic agents to tumors effectively with minimum damage to normal cells. In the past decades, the advantages of nanopreparations such as liposomes, micelles, nanoparticles, etc. over conventional therapies have been very well defined. Despite all efforts, only a small portion of the administered dose reaches the tumor site for its intended effect. Now, research has been focused on much more complicated delivery systems with less side effects, better targetability, enhanced intracellular penetration and stimuli-sensitivity. As a general concept, the effectiveness of conventional chemotherapeutics are considerably limited by systemic cytotoxicity, inability of bypassing biological barriers, poor biodistribution, nonspecific delivery and development of drug resistance. Engineering of stimuli-sensitive nanopreparations bases on particular characteristics of the tumor microenvironment such as reduced pH, increased local temperature, altered enyzmes/redox status, and scopes drug delivery systems that respond these specific differences. Glutathione (GSH) is the main reducing agent and the major thiol-disulfide redox buffer of the cells. Blood is generally an environment in which disulfide exchange reactions are minimal. Remarkably, glutathione concentration in a tumor mass is about 100-fold higher than the extracellular level of in normal tissues. This extreme concentration difference makes GSH a potential candidate stimulus for drug delivery to tumors especially in certain types of cancers. Disulfide bonds are increasingly under research as redox-responsive linkers for those drug delivery systems because GSH can easily breaks that bond. Following the reduction of the disulfide bonds, a rapid disruption of the nanocarriers results in the release of cancer therapeutics or siRNA. For this purpose, a redox responsive micellar nanocarrier PEG-ss-PE-PEI was synthesized providing redox potential-activated green fluorescent protein (GFP) silencing in vitro.
Figure : Stimuli-responsive drug targeting strategies via nanopreparations
Hanan Fael
Koç University, Turkey
Title: Poly (2-ethyl-2-oxazoline) as an alternative to poly (vinylpyrrolidone) in solid dispersions for solubility and dissolution rate enhancement of drugs
Time : 13:50-14:15

Biography:
Hanan Fael has her expertise in enhancing the bioavailability of poorly soluble drugs. She has worked on drug cocrystals at Barcelona University as an approach to enhance the solubility and dissolution of poorly soluble antibiotics. At the Koç University, her research focused on solid dispersion formation of drug with polymers as alternative technique to enhance the solubility and dissolution of a poorly soluble anti-diabetic drug. She has years of experience in Research and Teaching at Aleppo University and other educational institutes in Syria.
Abstract:
Poly (2-ethyl-2-oxazoline) (PEOX), a biocompatible polymer considered as pseudo polypeptide, was introduced as a potential alternative to the commonly used polymer, poly (vinylpyrrolidone) (PVP) for the preparation of solid dispersion with a poorly soluble drug. Glipizide (GPZ), a BCS class II model drug, was selected for solubility and dissolution rate study. GPZ-polymer solid dispersions and physical mixtures were characterized and investigated by X-Ray diffractometry; differential scanning calorimetry, scanning electron microscopy, and Fourier transform infrared spectroscopy. The impact of polymers on crystal nucleation kinetics was studied and PEOX exhibited strong inhibitory effect compared to PVP. Solubility and dissolution behaviour of the prepared solid dispersions and their physical blends were in-vitro examined and evaluated. A significant enhancement in glipizide solubility was obtained with PEOX compared to the pure drug and solid dispersion with PVP. A big improvement in the intrinsic dissolution rate (45 times) and dissolved amount of glipizide (58 times) was achieved with PEOX in FaSSIF (fasted state simulated intestinal fluid), against comparable enhancement observed with PEOX and PVP in phosphate buffer at pH 6.8. Lower molecular weight of PEOX-5K (5,000 g/mol) was found to be superior to higher molecular weight PEOX-50K (50,000 g/mol) in the improvement of dissolution behaviour. The findings of this study with glipizide as a model drug introduce lower molecular weight PEOX as a promising polymeric carrier toward better oral bioavailability of poorly soluble drugs.
Anselmo J Otero-Gonzalez
University of Havana, Cuba
Title: Chemical derivatives of Cm-p5, a mollusc-derived peptide, enhanced its antifungal properties and improved significantly its antibacterial activity in vitro
Time : 14:15-14:40

Biography:
Anselmo J Otero-Gonzalez is a presently working as a Microbiologist at the Havana University. He completed his PhD from National Centre for Scientific Research, Havana (1978) and Doctorate in Science from Havana University (1987). In 2008, he began working as a Senior Researcher at the Antimicrobial Peptide Lab, Havana University and sebsequently at (1981) Uppsala Separation School, Biomedical Centre University of Uppsala, Sweden, (1983) Department of Genetics, Pennsylvania University, Philadelphia, USA, (1991) European Collection of Animal Cell Cultures, Porton Down, Salisbury, (1992)Swedish Centre of Disease Control, Stockholm, Sweden, (2000) Harvard School of Public Health, Harvard University, Boston, USA, (2011-12) Harvard Medical School, Boston, USA and (2008) Bioorganic Department, Leibniz Institute for Plant Biochemistry, Halle (Saale), Germany. He has published 90 articles and 145 abstracts.
Abstract:
Antimicrobial peptides are an essential part of the first line of defence against microbial pathogens in many organisms. Current treatments for fungal infections are limited by drug toxicity and pathogen resistance. Cm-p5 (SRSELIVHQRLF) has a significant fungistatic activity against pathogenic Candida albicans. Cm-p5 was characterized by circular dichroism and nuclear magnetic resonance revealed an a-helical structure in membranemimetic conditions and a tendency to random coil folding in aqueous solutions. Additional studies modeling Cm-p5 binding to a phosphatidylserine bilayer in silico and isothermal titration calorimetry using lipid monophases demonstrated that Cm-p5 has a high affinity for the phospholipids of fungal membranes (phosphatidylserine and phosphatidylethanolamine), only moderate interactions with a mammalian membrane phospholipid, low interaction with ergosterol, and no interaction with chitin. Adhesion of Cm-p5 to living C.albicans cells was confirmed by fluorescence microscopy with FITC-labeled peptide. In a systemic candidiasis model in mice, intraperitoneal administration of Cm-p5 was unable to control the fungal kidney burden, although its low amphiphaticity could be modified to generate new derivatives with improved fungicidal activity and stability. Chemical and sequential derivatives have been synthetized to enhance the antimicrobial spectrum of Cm-p5. A cycled derivative (cys-cys Cm-p5) improved the minimal inhibitory concentration of the parental peptide from 10 to 5 µg/mL against Candida albicans. Cys-Cys CM-p5 was not toxic for human macrophages, the major host cell for the bacterial pathogen M. tuberculosis. Antimicrobial activity against extracellular, virulent M. tuberculosis reached >80% at 300 µg/ml concentration and was nearly as efficient as the first line antimicrobial drug rifampin.
Fabiola Porta
University of Basel, Switzerland
Title: Design of nanosized drug delivery materials: Determinant biophysical aspects for patient oriented nanotherapy
Time : 14:40-15:05

Biography:
Fabiola Porta graduated in Chemistry from Leiden University, the Netherlands, in 2012 with a thesis focused on the synthesis and characterization of silica mesoporous nanomaterials as drug delivery systems. Since 2013, she has joined University of Basel as a Research Associate in the field of Drug Delivery. At the University of Basel, she is a Lecturer of Bio-Nanomaterials in the Drug Delivery lectures series of the Master of Drug Sciences. In 2017, she has been awarded the “Novartis University of Basel Excellence Scholarships for Life Science”. She is now continuing her work on bio-nanomaterials as a Principal Investigator in the group of Biopharmacy of the Department of Pharmaceutical Sciences.
Abstract:
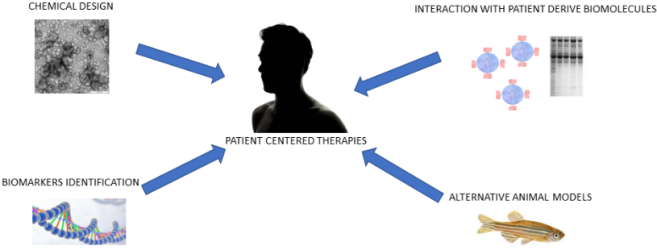
The development of nanosized pharmaceutical materials is nowadays facing several challenges in order to design nano drug delivery systems to be applied in patients. The design of biocompatible backbone, the identification of pathological biomarkers for targeted disease nanotherapy and the investigation of potential interaction of the nanomaterial with patient derived biomolecules are the main aspects in which science is striving. In our research group, we have defined a new strategy in which the main aspects of nano drug delivery design are tackled together. Identification of pathological biomarkers recurrent in cancer has been chosen as a target strategy for our systems. Cathepsin B, a fundamental enzyme for the maintenance of the cellular homeostasis, has an increased expression in ovarian cancer. Using enzyme cleavable nanoparticles, we have developed a targeted nanomedical approach to deliver bioactive compounds to ovarian cancer cells. However, the design of targeted nanotherapy has to be implemented with further studies considering the potential interactions of nanoparticles with patient derived molecules. For examples, proteins present in the blood plasma can significantly change biophysical properties of nanomaterials. For instance, the deposition of proteins on the surface of nanovesicles can lead to nanomaterials with increased diameters and different surface charges, which are differently interacting with the targeted cells. Through our study, we have deepened our knowledge investigating the formation of a biocorona on polymeric nanovesicles and studying the difference in cellular uptake after the protein layer formation. We have shown that the biocorona formation is deeply influencing the cellular uptake of nanovesicles of cancer cells. Therefore, we believe that this is a fundamental biophysical parameter for the development of novel pharmaceutical nanosized materials. The implementation of our investigations with alternative animal model, render our study approach very complete and more patient oriented.
Figure : General representation of innovative strategy to tackle drug delivery design. From the chemical design of biocompatible nanoparticles to identification of novel biomarkers it is possible to design smart responsive nanosized pharmaceutical materials. In addition, implementation of the nanosystems with studies in alternative models and patient derived biomolecules allows the characterization and the fine tuning of biophysical properties for a patient centered nanotherapy.
Felix-Martin Werner
Euro Academy Pößneck, Germany
Title: Treatment of extrapyramidal symptoms in patients treated with antipsychotic drugs
Time : 15:05-15:30

Biography:
Felix-Martin Werner studied Medicine at the University of Bonn. He has been working as a Medical Teacher in the formation of Geriatric Nurses, Occupational Therapists and Assistants of Medical Doctors at the Euro Academy in Pößneck since 1999. He has been doing scientific work at the Institute of Neurosciences of Castilla and León in Salamanca in Spain since 2002. With Professor Rafael Coveñas, he assisted over 30 national and nine international congresses and published over 40 reviews about neural networks in neurological and psychiatric diseases. Since 2014, he has been serving the Editorial Board of the Journal of Cytology and Histology.
Abstract:
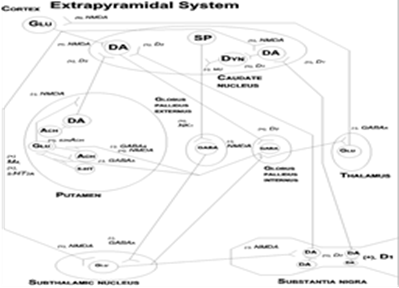
Extrapyramidal symptoms, for example dyskinesia, parkinsonism, akathisia or dystonia can occur in schizophrenic or schizoaffective patients treated with antipsychotic drugs. Second-generations antipsychotic drugs such as risperidone, olanzapine, quetiapine mostly are D2 and 5-HT2A antagonists and can cause these movement disturbances as a consequence of the D2 receptor blockade. In the mesolimbic system, the prefrontal cortex and the hippocampus, dopamine and serotonin hyperactivity and GABA and glutamate hypoactivity are induced by susceptibility genes found in schizophrenia and schizoaffective disorder. The function of neurotransmitters and neuropeptides in the extrapyramidal system are reviewed. Neural networks in the extrapyramidal system and the mesolimbic system are described. In the extrapyramidal system, the neurotransmitter balance between D2 dopaminergic and muscarinic cholinergic neurons and between presynaptic GABAergic and excitotoxic glutamatergic neurons is altered by the second-generation antipsychotic drugs. Possible treatments of extrapyramidal symptoms induced by antipsychotic drugs are M4 antagonists, GABAA agonists and NMDA antagonists. The adverse effects of these additional drugs are presented. Some new antipsychotic drugs such as aripiprazole and cariprazine less often and to a lesser extent cause extrapyramidal symptoms, because they have a partial agonism at the D2 receptor.
Amélia C F Vieira
University of Coimbra, Portugal
Title: Diclofenac-β-cyclodextrin for colonic drug targeting: From synthesis to in vivo performance in rats
Time : 15:50-16:15

Biography:
Amélia C F Vieira has completed her PhD from Faculty of Pharmacy, University of Coimbra. Her PhD thesis entitled, “Synthesis of diclofenac-cyclodextrin conjugates for colon delivery” resulted in a European Patent Application and in three publications in reputed journals. She works as Pharmaceutical Development Technical Specialist at Labor Qualitas-Tecnimede Group.
Abstract:
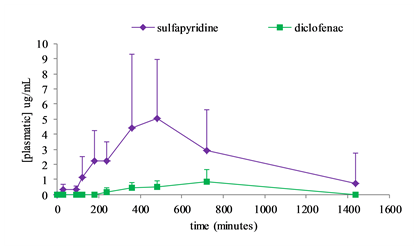
A new conjugate of diclofenac with β-cyclodextrin through an ester linkage has been synthesized. Diclofenac is a non-steroidal anti-inflammatory drug, its targeting to the colon would provide a suitable tactic for the management of arthritis pain by chronotherapy with simultaneous circumvention of the adverse gastric effects associated with the free drug. β-cyclodextrin is a cyclic oligosaccharide that can function as a carrier to develop colon-targeted prodrugs through the synthesis of a suitable covalent linkage with a drug. However, it was required to exploit various strategies to form the required ester linkage between the diclofenac and β-cyclodextrin. Only the nucleophile substitution of mono-6-tosyl-β-cyclodextrin under microwave irradiation allowed an efficient successful synthesis. The conjugate was identified by proton nuclear magnetic resonance (1H-NMR) spectroscopy and matrix-assisted laser desorption/ionization (MALDI) spectra. Stability of the diclofenac-β-cyclodextrin conjugate were carried out in human fecal slurries and in simulated gastric and intestinal fluids. Results demonstrated that the conjugate released diclofenac at the level of the lower intestine, and exhibited good stability in the upper gastrointestinal tract. As a proof-of-concept, a comparative in vivo study in fasted rats was performed by oral administration of a suspension of prodrugs, diclofenac-β-cyclodextrin and a well-known prodrug, sulfasalazine to a group of rats. A lag time between oral intake of prodrugs and the appearance of the respective drugs in plasma was observed. Overall, this study confirms the in vivo ability of our newly cyclodextrin prodrug to target and release diclofenac specifically in the colon.
Figure : Concentration-time profiles of diclofenac and sulfapyridine in Wistar rats after simultaneous oral administration of diclofenac-β-cyclodextrin (88.5 mg/kg) and sulfasalazine (100 mg/kg). Each bar represents mean±SD (n = 7).
Kostas D Demadis
University of Crete, Greece
Title: Self-sacrificial metal-organic hybrid materials for controlled release of bisphosphonate osteoporosis drugs
Time : 16:40-17:05

Biography:
Kostas D Demadis is a Full Professor in the Department of Chemistry, University of Crete, Greece and Head of the Crystal Engineering, Growth & Design Laboratory. His research group is interested in a number of research areas such as coordination polymers with emphasis on metal phosphonate MOFs, functional polymers, silicon chemistry (modeling of biosilicification mechanisms), water treatment issues (mineral scale inhibition, corrosion control, metal ion absorption), controlled release of active ingredients (in particular bisphosphonate drugs), “green” chemistry, and hybrid polymeric materials for cultural heritage protection. He has published ~150 papers in peer reviewed journals, about a dozen chapters in books, four books, and is the inventor of two patents.
Abstract:
Osteoporosis is among the well-known bone diseases (other are osteoarthritis, multiple myeloma, Paget’s disease etc.), which burdens millions of people compromising patients’ quality of life. The recommended pharmaceutical treatment is the use of bis-phosphonates (BPs, a.k.a. “-dronates”). Their success in mitigating osteoporosis, notwithstanding these “-dronate” drugs present a number of challenges including fast excretion, and numerous side-effects, such as osteonecrosis of the jaw, hypocalcemia, esophageal cancer, ocular inflammation, atrial fibrillation, etc. Nevertheless, the main drawback of BPs is their limited oral bioavailability. It is, therefore, imperative to design and fabricate “smart” systems that allow controlled delivery of the active BP agent, which will depend on the patient’s needs and idiosyncrasies. In this presentation, we discuss drug delivery systems that are based on metal–organic frameworks (MOFs). MOFs are well defined crystalline materials that possess an “inorganic” part (the inorganic metal ion) and an “organic” part, a molecule that can form coordinating bonds with the metal ion. In these materials, we have used biologically acceptable inorganic metal ions (eg. Ca2+) and bisphosphonates as the organic portion. These materials have been synthesized, characterized, and studied for the self-sacrificial release (by pH-driven dissolution) of the bisphosphonate active ingredient. Several such materials were prepared with a variety of bisphosphonate drugs. They exhibit variable release rates and final % release, depending on the actual structure of the metal-bisphosphonate material.
Ali Awad Hamoud Al-jeboory
Uruk University, Iraq
Title: Haloxylon as an alternative nanoscience medicine in treatment of cancer
Time : 17:05-17:30

Biography:
Ali Awad Hamoud Al-jeboory has completed PhD at the age of 30 years from strathclyed University. He is the professor of Uruk Pharmacy College, Uruk Private University. Ali Awad Aljeboory has published 50 papers and has had 5 discoveries of new drugs from natural products antihypertensive and heart tonic. In addition serving as an editorial board member of Al-mustanseriah College of medicine journal. Also supervised 20 PhD graduated students in pharmacology, published two books in natural pharmacology and pharmacy. He was the director of Kufa College of medicine, head of the department of pharmacology, pharmacognosy in scientific research center, Head of the department of pharmacology and Therapeutics College of medicine Baghdad University. Currently working as Director of Uruk Pharmacy College.
Abstract:
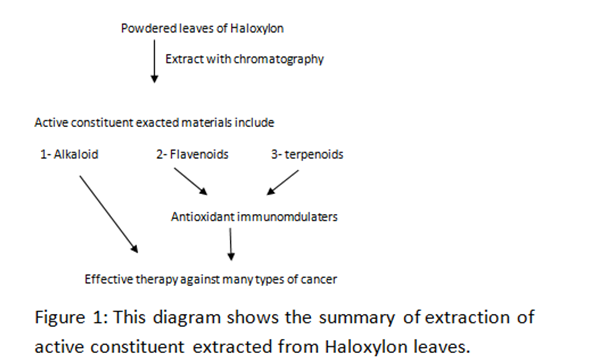
Cancer is a disease that affects about millions of Iraqi people in all age groups and both sexes. Most of current chemotherapeutic agents on the market are low index and lack of specificity with unwanted effects and cause significant damage to non-cancer tissues. Additionally, they are expensive. In Iraq, the cancer level increased because of war using dirty weapons and low care of health and poor nutrition so pushed us to look for a new drug resources, cheap and easy to get from natural medicine. However we have looked to traditional medicine which has less resistance and effective in treatment of cancer with fewer side effects. Among screening of many traditional plants we found that some of these medicinal plant of a good efficient and significant value in treatment of cancer especially after extraction of active constituent from their leaves, fruits, stems and roots. Among these are Haloxylon, Loranthus ferrugineus and Loranthus europaeus. In addition, using gold which one of inert materials. The activity of these natural materials when mixed with vincristine has shown tremendous result. This potentiation lead to decrease the dose and decrease the side effect of vincristine the structure and tunable surface functionality of these agents allows for the encapsulation of multiple entities may render them ideal drug deliver agents for various anticancer drugs which have ability to arrange excretion mode from body as a function of nanoscale diameter. From these result especially with Haloxylon active constituent may act as a new anticancer drug delivery or a new anticancer drug with high significant activity even with very low doses with low price and available resources from Iraqi desert, thus, this multifunctional unique small nanoparticulate has the potential to detect diseases, deliver medications which could change current scenario of cancer research and could be help in diagnosis at the same time. The extraction of active constituent materials includes alkaloid, flavonoids and terpenoid by using chromatography method (using a thimble of Soxhlet) with different organic solvents. The alkaloid kills the cancer cells and flavonoids with terpenoids act as antioxidant which potential the anticancer activity of these natural agents.
- Nanoparticulate Drug Delivery Systems | Biomedicine and Pharmacotherapy Pharmaceutical Nanotechnology | Nanomedicine and Nanotechnology
Location: Zieten

Chair
Hyungil Jung
Yonsei University, South Korea

Co-Chair
Kostas D Demadis
University of Crete, Greece
Session Introduction
Margitta Dathe
Leibniz-Forschungsinstitut für Molekulare Pharmakologie, Germany
Title: Peptide-modified micelles and liposomes: Carriers for xenon hyper-CEST MRI of blood brain barrier endothelial cells
Time : 11:30-11:55

Biography:
Margitta Dathe studied Physics at the Humboldt University of Berlin and completed her PhD in 1978 from the Academy of Sciences of the GDR. Since 1999, she has been working as Head of the Peptide-Lipid Interaction Research Group of the Leibniz Research Institute of Molecular Pharmacology. Her research interest is focused on targeting, cellular uptake promoting peptides and lipid-based carrier systems as well as on antimicrobial peptides. She has published more than 100 papers in reputed journals
Abstract:
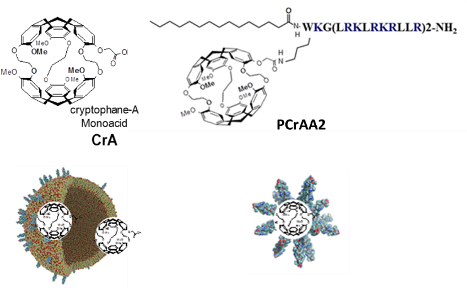
Selective imaging of pathological areas and targeted drug delivery are crucial for efficient diagnostics and therapy. Drug delivery to the brain is a particular challenge. We generated highly cationic lipopeptides that form micelles and bind to liposomes. Cargos, covalently bound or incorporated into such carriers are selectively transported into blood brain barrier endothelial cells. Basis for the selective uptake of the different systems is the activation of clathrin-mediated endocytosis, a process which is not addressed in other vessel endothelial cells. Here we present the development of peptide-modified micellar and liposomal carriers for the selective transport of cryptophane-A (CrA) into human brain capillary endothelial cells. Chemical exchange saturation transfer with hyperpolarized xenon nuclei (Hyper-CEST) allows highly sensitive detection of supramolecular cages such as CrA in non-invasive Magnetic Resonance Imaging (MRI). Incorporation into liposomes distinctly reduced the toxicity of the hydrophobic CrA and a one nanomolar concentration generated sufficient contrast to distinguish between brain capillary and aortic endothelial cells. Covalent attachment did not influence the micelle characteristics and provided additional advantages as it results in high local cage concentration and allows more reliable quantification of the signal molecule. The peptide-modified carriers combine a high selectivity for human brain capillary endothelial cells with the great sensitivity of Xe Hyper-CEST MRI and might be a promising MRI tool.
Scheme of peptide-tagged CrA-loaded liposomes and PCrAA2 micelles for Xenon Hyper-CEST MRI
Ruba Bnyan
Liverpool John Moores University, UK
Title: Transfersomes as novel carriers for sustained buccal delivery of local anaesthetic
Time : 11:55-12:20
Biography:
Ruba Bnyan is a PhD student in the Formulation and Drug Delivery Research Group, School of Pharmacy and Biomolecular Sciences, Liverpool John Moores University. Her research interests are generally in designing drug delivery system, nanotechnology and pharmaceutical technology, focus being on preparing non-invasive delivery systems for several routes of administrations.
Abstract:
The aim of this work was to design and develop a delivery system to treat dental and buccal pain. Transfersomes show many advantages as delivery vesicles such as their ability to deform and pass through small pores between cells, and to encapsulate drugs with a wide range of solubilities and molecular weights. The rationale of preparing sustained release transfersomes of local anaesthetic (LA) was mainly to reduce the frequency of administration and enhance the safety profile of LA by producing a localized effect. Transfersome preparation parameters were optimized using a Taguchi design of experiment (DOE) in terms of phospholipid to edge activator (EA) ratio, type of EA and type of lipid. The delivery systems were characterised for vesicles size, polydispersity index (PDI), charge, and entrapment efficiency (EE). They were generally less than 200 nm in size with a low PDI. The %EE varied as the formulation parameters changed, but was generally between 44-50%. Analyzing the data by Taguchi DOE showed that the effects of factors on both size and %EE were in the following rank: EA type˃lipid: EA ratio˃lipid type. Samples that showed higher encapsulation with smaller vesicles size were chosen for further studies. In vitro release studies were performed using a dialysis bag (3-5 kDa) as a donor compartment, which was sealed and placed in a receptor compartment containing PBS. The system was stirred at 250 rpm and incubated at 37oC. Initial in vitro release results showed a sustained release over 72 hours.
Janina-Miriam Noy
The University of New South Wales, Australia
Title: Direct polymerization of the novel arsenic drug PENAO to obtain polymeric nanoparticles for the treatment of sarcoma
Time : 12:20-12:45

Biography:
Janina-Miriam Noy has completed her Bachelor’s and Master’s Degree at the Heinrich-Heine University in Düsseldorf (Germany) before she relocated to Sydney (Australia) in 2013. She worked as a Research Assistant for two years at the Centre of Advanced Macromolecular Design (CAMD) at the University of New South Wales, focusing on the development of new stimulus-responsive materials for ‘smart’ drug delivery systems. Since August 2015, she is undertaking her PhD with the focus on the delivery of novel organicarsenical anti-cancer agents within polymeric nanoparticle formulations. She particularly investigates her arsenic containing drug-delivery systems towards sarcoma cells.
Abstract:
Recent investigations have shown the anti-cancer efficiency of PENAO, a second generation hydrophilic organoarsenical, towards a range of cancer cell lines and in a phase I/IIa dose escalation study in patients with solid tumours. However, the efficacy of PENAO – like most metal-based drugs – is limited by several factors such as high systemic toxicity, development of drug resistance, and rapid deactivation by complexation with proteins or oxidation reactions. Conjugation of drugs to a nanocarrier is an alternative strategy that overcomes many of these limitations. Polymeric nanoparticles for the delivery of chemotherapeutics has been widely proposed to prolong circulation time in the blood stream, to increase the specific retention in solid tumour tissue (enhanced permeability and retention (ERP) effect), and to avoid the recognition by the mononuclear phagocyte system. Herein, the direct synthesis of polymeric micelles, based on the novel arsenic drug PENAO is presented. PENAOs arsenous acid residue remains active when incorporated into a polymer matrix and conjugates to small mono and closely spaced dthiols, showing no significant difference in efficiency between PENAO containing polymers, PENAO containing nanoparticles and PENAO itself. Furthermore, the more stable micelle structures induce apoptosis in sarcoma cells and enhanced cytotoxicity and cellular uptake compared to the free drug. As a result, PENAO containing nanoparticles show great potential for further investigations into the biomedical arena and increasing the concentration of PENAO within the polymeric nanoparticle could improve antitumor efficiency, which leads to an auspicious outcome towards sarcoma cells.
Ilayda Acaroglu Degitz
Yeditepe University, Turkey
Title: Paclitaxel release from magnetite crosslinked polypropylene fumarate nanoparticles
Time : 14:45-15:10

Biography:
Ilayda Acaroglu Degitz has completed her MSc from Yildiz Technical University in 2013 and has been pursuing her PhD Degree at Yeditepe University in the Chemical Engineering Department since 2014. She has been working on nanoparticles, magnetic nanoparticles, drug delivery systems, drug and polymer conjugates. She has published two papers on nanoparticles. She is working as a Teaching and Research Assistant at Yeditepe University
Abstract:
In the treatment of cancerous cells, the most common treatment, chemotherapy, involves high toxicity and non-specific targeting in the body which leads to both cancerous and non-cancerous cells, inside and outside the tumor becoming damaged or destroyed due to the agents spreading throughout the body which further limit the productivity. Therefore, attempts are more focused on targeting cancer therapeutics, thus reaching the objective of developing new carrier systems with both existing and new drugs. Magnetic drug delivery using drug carriers such as biodegradable polymers, is a very efficient method to target the drug to a localized disease site in the body. The aim of this study was to develop a new magnetic drug delivery system. For this purpose, magnetic nanoparticles were incorporated in biocompatible polymers namely polypropylene fumarate (PPF) which was crosslinked with an N-vinyl pyrrolidone (NVP) using photo initiated miniemulsion polymerization method. FTIR spectroscopy was used to confirm the crosslinking of PPF with NVP, and swelling tests were used to determine the percentage of crosslinking. Then the morphology and size of crosslinked polymer particles with and without magnetite were determined by scanning electron microscopy (SEM). Crosslinked polymer nanoparticles with embedded magnetic nanoparticles were loaded with paclitaxel (anticancer drug). The amount of drug released from crosslinked polymer nanoparticles with and without magnetite was determined using high-performance liquid chromatography (HPLC) and these carriers were found to release all the encapsulated drugs in less than one month, before the polymer nanoparticles were degraded. Thus, it suggests the drug release to be diffusion controlled.
Hassan Bardania
Yasuj University of Medical Sciences, Iran
Title: Cell targeting small peptides as smart ligands for targeted drug delivery
Time : 15:10:15:35
Biography:
Hassan Bardania has completed his PhD from Tarbiat Modares University, Iran. He has published more than 10 papers in reputed journals.
Abstract:
Targeting ligands are used in drug delivery to decrease the side effects and increase the bio-availability of drugs. Small peptides with high affinity for special receptors have attracted more attention as a new class of ligands to deliver specifically therapeutic and diagnostic agents. They are typically identified by using phage display and chemical synthetic peptide library methods. In comparison with other conventional ligands such as antibodies, these ligands have several advantages including easy synthesis, smaller physical sizes, lower immunogenicity and cytotoxicity and their simple and better conjugation to nano-carriers and therapeutic or diagnostic agents. In our research, we have used small peptides as a targeting ligand to deliver therapeutic agents to activated platelets and cancerous cells. In our previous study, we used nanoliposoms modified with a motif of RGD peptide to deliver eptifibatide (as an anti-platelet drug) to activated platelets. The in vitro and in vivo results showed that encapsulation of eptifibatide into RGD-modified nanoliposomes significantly improved platelet aggregation inhibitory activity of drugs compared to free drugs. In our next study, we will be using small peptides as targeting ligands for drug targeting to cancerous cells.